Method of molecular orbitals. Lecture_4_Chemistry Method of molecular orbitals n2
In all discussions about the formation of covalent bonds and the geometric structure of molecules according to valence bond method the question was bypassed: what causes atoms to form covalent bonds? For this it is necessary to turn to the consideration of energy.
In atoms, electrons ( ē ) exist in allowed energy states – on A languid ABOUT rbitals.
Similarly, in molecules, ē exist in allowed energy states, which are called M molecular ABOUT rbitals and, because molecules are more complex than atoms, then => MO is more complicated than AO.
The molecular orbital method (MMO) has a greater predictive power, where:
- a molecule is considered as a single system of nuclei and electrons;
-electrons are in common use by all the nuclei of the atoms that form the molecule;
Thus, the MO method considers a chemical bond as a multicenter and multielectron . In this case, for an approximate solution of the Schrödinger equation, the wave function psi ψ , corresponding to the MO, is given as a linear combination of AO, i.e., as the sum and difference of atomic wave functions with variational coefficients (c 1 , s 2), which determine the share of AO participation in the construction of the MC or indicate the share of their contribution to the overlap of electron clouds.
When adding AO, the formation of MO: ψ + = с 1 ψ 1 + с 2 ψ 2,
When AO is subtracted, MO is formed: ψ - = s 3 ψ 1 – s 4 ψ 2.
MO as well as AO are characterized by quantum numbers:
n the main l side, m l magnetic, determining their energy, number and orientation in space: AO - s p d f, MO - σ π δ φ .
The resulting method was called the Linear Combination of Atomic Orbitals (LCAO MO). In the LCAO MO method, in order to form a stable molecular orbital, it is necessary that
1) the energies of the atomic orbitals were close to each other;
2) so that their symmetry does not differ much. When these 2 requirements are met, the coefficients c 1 and c 2 must be close in their values, and this ensures poppy maximum AO overlap.
If MO is formed, the energy of which going down relative to the AO energies, then such an MO is called binding . The wave function corresponding to the binding MO is obtained by adding wave functions with the same sign ψ + = c 1 ψ 1 + c 2 ψ 2 . In this case, the electron density is concentrated between the nuclei, and the wave function takes on positive value.
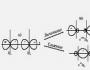
When subtracting the wave functions ψ - = с 3 ψ 1 – с 4 ψ 2 the MO energy increases. This orbital is called loosening . The electron density in this case is located behind the nuclei, and between them is equal to zero. The wave function in the two formed electron clouds has opposite signs, which is clearly seen from the formation scheme of the bonding and loosening orbitals shown in (presentation Fig. 24,25):
Rice. 24. Scheme of the formation of bonding and loosening molecular orbitals.
When the AO (presentation in Fig. 26) of one of the atoms, due to a large difference in energy or symmetry, cannot interact with the AO of another atom, it passes into the energy scheme of the MO of a molecule with the energy corresponding to it in the atom. This type of orbital is called non-binding .
Classification of orbitals on σ or π (presentation Fig.27, 28) is produced in accordance with the symmetry of their electron clouds in a similar way σ - And π -bonds in the method of valence bonds:
σ-orbital has such a symmetry of the electron cloud, in which turning it around the axis connecting the nuclei by 180 0 leads to an orbital that is indistinguishable from the original in shape. The sign of the wave function does not change in this case;
π orbitals- when it is rotated by 180 0, the sign of the wave function is reversed.
Hence it follows that
s-electrons atoms, when interacting with each other, can form only σ-orbitals,
and three p-orbitals atom - one σ- and two π orbitals, and σ - orbital occurs when interacting p X atomic orbitals, and π orbital- when interacting p Y And pZ. Molecular π-orbitals are rotated relative to the internuclear axis by 90 0 . Here there is a complete analogy with the method of valence bonds (presentation Fig. 29).

Rice. 29. Scheme of the formation of binding and loosening MOs
for 2p atomic orbitals.
In order to distinguish connection and res squishy orbitals each
from a friend, as well as their origin, the following notation. The bonding orbital is denoted by the abbreviation "sv", located at the top right after the Greek letter denoting the orbital, and loosening - respectively "razr".
Another designation is adopted: an asterisk * marks the antibonding orbitals, and without an asterisk, the bonding ones. After the designation of MO, the designation of AO is written, to which the molecular one owes its origin, for example, π razr 2p y. This means that the π-type molecular orbital, loosening, was formed during the interaction of 2 p y -atomic orbitals (Fig. 29):
When considering the electronic structure of a molecule from the point of view of the molecular orbital method (MMO), one should be guided by the following rules:
1. Electrons in a molecule, as in an atom, occupy the corresponding
orbitals that are characterized with its own set of quantum numbers;
2. The number of formed MOs is equal to the number of initial AOs;
3. The energies of bonding MOs are lower than the energies of AOs, and the energies of loosening MOs are higher than the energies of AOs that accept bonds in formation.
4. Electrons are placed on the MO according to the principle of least energy (right. Klechkovsky), Pauli's principle, Hund's rule.
5. A chemical bond between atoms is formed if the number electrons there are more electrons on loosening MO.
6. For molecules, formed by atoms of one chemical element
(homonuclear), the gain in energy due to the formation of a bonding MO is compensated by an increase in the energy of the loosening MO.
On the energy diagram, both orbitals are located symmetrically
regarding atomic orbitals (presentation fig.32, 33):

Rice. 33. Energy diagram of molecular orbitals
for homonuclear molecules (on the example of a hydrogen molecule)
7. For molecules, formed by atoms of different elements(heteronuclear), bonding orbitals closer in energy to orbitals more electronegative atom (B), A loosening - closer to the orbital less electronegative atom (A). The difference in the energies of the initial atomic orbitals (segment b) is equal to ∆ of the bond polarity; this difference is a measure of the ionicity of the bond. The difference in energy between the bonding orbital and the atomic orbital (segment c) of the more electronegative atom determines the covalence of the bond. (presentation fig.34):

Rice. 34. Energy diagram of molecular orbitals
for a heteronuclear molecule.
8. Chemical bond multiplicity is equal to half the difference between the number of electrons located in bonding orbitals and the number of electrons in loosening orbitals: n = ½ (Nbond – Nrel).
When describing a molecule in terms of MMO, we will adhere to the following plan (presentation in Fig. 35):
1. Determine which AO overlap and form MO
2. Construct an energy diagram of the MO of a molecule (ion)
3. Distribute electrons over the MO in accordance with the principle of least energy, the Pauli principle and the Hund rule
5. Assume the magnetic properties of a molecule (ion)
6. Compare the ionization energy of the molecule (ion) and the initial atoms
7. Spectral properties of a molecule (ion)
For example, let's analyze the energy diagrams and the electronic structure heteronuclear And homonuclear molecules and ions formed by two atoms of elements first and second periods of the Periodic system .
Elements first period (presentation Fig.36) the valence orbital is the 1s-orbital. These two atomic orbitals form two σ-molecular orbitals - bonding and loosening.
Let us consider the electronic structure of the molecular ion H 2 + . It has one electron, which will occupy the more energetically favorable s-bonding orbital. In accordance with the rule for counting the multiplicity of bonds, n \u003d 0.5, and since there is one unpaired electron in the ion, H 2 + will have paramagnetic properties. The electronic structure of this ion will be written by analogy with the electronic structure of an atom as follows: σ bond 1s 1 .
The appearance of a second electron in the s-bonding orbital will lead to an energy diagram describing the H 2 hydrogen molecule, an increase in the bond multiplicity to unity, and diamagnetic properties. An increase in the multiplicity of communication will entail and increase in dissociation energy H 2 molecules and shorter internuclear distance compared to that of the hydrogen ion. The electronic structure of H 2 can be written as follows: σ bond 1s 2 .

Energy diagrams of the elements of the I period (presentation fig. 34)
The diatomic molecule He 2 will not exist, since the four electrons present in two helium atoms will be located on the bonding and loosening orbitals, which leads to a zero multiplicity of bonds. But at the same time, the He 2 + ion will be stable and the bond multiplicity in it is 0.5. Just like the hydrogen ion, this ion will have paramagnetic properties.
Elements second period (presentation Fig. 37) four more atomic orbitals appear: 2s, 2p X, 2p Y, 2p Z, which will take part in the formation of molecular orbitals.
The energy difference between 2s and 2p orbitals is large, and they will not interact with each other to form molecular orbitals. This energy difference will increase as you move from the first element to the last. In connection with this circumstance electronic structure of diatomic homonuclear molecules of elements of the second period will be described by two energy diagrams (presentation in Fig. 38), which differ in the order in which they are located σ bond 2p x And π sv 2p y,z electrons.
With the relative energy proximity of the 2s- and 2p-orbitals observed at the beginning of the period, including the nitrogen atom, the electrons located in the σ res 2s- and σ bonds 2p x orbitals repel each other. Therefore, π bonds 2p y and π bonds 2p z -orbitals turn out to be energetically more favorable than σ bonds 2p X -orbital. On fig. 38 shows both diagrams.
Since the participation of 1s electrons in the formation of a chemical bond is insignificant, they can be ignored in the electronic description of the structure of molecules formed by elements of the second period.
Presented in fig. 38 energy diagrams, confirmed by spectroscopic data, show the following order of molecular orbital placement with increasing energy from Li 2 to N 2 inclusive:
a) a from O 2 to F 2:
σ bond 1s< σ разр 1s << σ связ 2s< σ разр 2s << σ связ 2p X < π связ 2p У = π связ 2p z < π разр 2p У < π разр 2p z << σ разр 2p X .
b) from Li 2 to N 2 inclusive:
σ bond 1s< σ разр 1s << σ связ 2s < σ разр 2s < π связ 2p У = π связ 2p z < σ связ 2p X < π разр 2p У < π разр 2p z << σ разр 2p X ;

Rice. 38. Energy level diagrams of diatomic molecules
with a significant and insignificant energy difference between the atomic 2s and 2p orbitals.
The second period of the system is opened by lithium and beryllium, in which the outer energy level contains only s-electrons.
Energy diagrams of the elements of the second period (presentation Fig.39, 41) from Li to F.

For these elements, the scheme of molecular orbitals will not differ in any way from the energy diagrams of molecules and ions of hydrogen and helium, with the only difference that for the latter it is built from 1s electrons, and for Li 2 and Be 2 from 2s electrons. The 1s electrons of lithium and beryllium can be considered as nonbonding, i.e., belonging to individual atoms. Here, the same patterns will be observed in changing the bond order, dissociation energy, and magnetic properties. The Li 2 + ion has one unpaired electron located on the σ bond of the 2s orbital - the ion paramagnetic. The appearance of a second electron in this orbital will lead to an increase in the dissociation energy of the Li 2 molecule and an increase in the bond multiplicity from 0.5 to 1. Magnetic properties will acquire diamagnetic character. The third s-electron will be located on the σ 2s-orbital, which will help reduce the bond multiplicity to 0.5 and, as a result, lower the dissociation energy. Such an electronic structure has a paramagnetic ion Be 2 + . The Be 2 molecule, just like He 2 , cannot exist because of the zero bond order. In these molecules, the number of bonding electrons is equal to the number of loosening ones!

Further filling of molecular energy levels for
diatomic homonuclear molecules and some ions of elements of the second
period is shown in (presentation Fig. 40,42,43). As can be seen from Figure 40, as bonding orbitals are filled, the dissociation energy of molecules increases, and with the appearance of electrons in loosening orbitals, it decreases. The series ends with an unstable Ne 2 molecule. The figure also shows that the removal of an electron from the antibonding orbital leads to an increase in the bond multiplicity and, as a consequence, to an increase in the dissociation energy and a decrease in the internuclear distance. The ionization of the molecule, accompanied by the removal of the binding electron, has the opposite effect.
Electronic formulas of 2 atomic molecules of elements of the second period:
Let us compare the energy diagrams of the following two pairs of molecules and
ions: O 2 +, O 2, N 2 +, N 2, shown in (presentation Fig. 40):

Rice. 40. Energy diagrams of diatomic molecules and ions
elements of the second period of the Periodic system.

The energies of the constructed molecular orbitals can be determined from the data of the absorption spectra of substances in the ultraviolet region. So, among the molecular orbitals of the oxygen molecule formed as a result of overlapping p-AO, two π connection living degenerate (with the same energy) orbitals have less energy than σ bond living I, however, like π*-cut sagging orbitals have less energy compared to σ*- res squirming orbital
In the O 2 molecule, two electrons with parallel spins ended up in two degenerate (with the same energy) π*-loosening molecular orbitals. It is the presence of unpaired electrons that is responsible for paramagnetic properties of the oxygen molecule, which will become noticeable if oxygen is cooled to a liquid state (presentation in Fig. 44). .
heteronuclear molecules. When describing the energy diagrams of the MO of heteronuclear molecules, the following rules (presentation Fig. 45):
1. The initial AO give different contributions to Esvyaz. and Erazr. MO.
2. Number of MO = number of AO; number of connections MO = number of res. MO = the number of AOs of the atom that has fewer of them.
3. AOs are effectively overlapped, the energy of which differs by no more than 20 eV.
4. AOs are effectively overlapped, the symmetry of which with respect to the internuclear axis is the same.
5. AOs that do not participate in the effective overlap transform into nonbonding MOs without a change in energy.
Of the diatomic molecules, the strongest is the nitrogen molecule, in which the bond multiplicity is three. It is logical to assume that for heteronuclear molecules and singly charged ions having the same number of electrons with N 2 - (14=7+7) - the bond multiplicity will be the same.
Molecules that have the same number of electrons in the same orbitals are called isoelectronic (presentation fig. 46).
Such molecules are CO, BF, BeNe and ions CN - , NO + , CF + , BO - (presentation Fig. 47). By analogy with the nitrogen molecule, they should have high dissociation energies. It is not difficult to draw such a conclusion by extending the scheme of MO of homonuclear molecules to heteronuclear ones.
In this case, it should be taken into account that the s- and p-orbitals decrease their energy with increasing nuclear charge, and the splitting between them in energy increases. Diagram (Shriver, Atkins presentation Fig. 43).
As a result, significant differences appear in the formation of molecular orbitals in some heteronuclear molecules from homonuclear ones. Let us illustrate this statement by the example of the NO+ ion and
CO molecules.

Rice. 47. Energy diagrams for the NO+ ion (a) and the CO molecule (b).
Since the charges of the nuclei of nitrogen (+7) and oxygen (+8) atoms differ by one, there is no significant difference in the energies of their atomic orbitals and the MO scheme NO+ ion will similar MO scheme nitrogen molecules N 2(Fig. 47a).
All p-orbitals of the oxygen atom (+8) are located lower in energy than the corresponding atomic orbitals of the carbon atom (+6), since the charge of the oxygen nucleus is two units higher (Shriver, Atkins presentation Fig. 43). The result of these energy differences will be a significant difference between the CO molecular orbitals of carbon monoxide and the molecular orbitals of the NO+ ion (Fig. 47b).
2s-orbital (presentation Fig. 48). oxygen is located significantly below the 2s-orbital of carbon, which results in their weak interaction, leading to the formation of a weakly binding σb-orbital, the energy of which practically does not differ from the atomic 2s-orbital of oxygen. At the same time, the energies of the 2p orbitals of oxygen and the 2s orbitals of carbon are close. This proximity leads to the formation of two σ sv -bonding and σ unbonding orbitals. If the upper occupied σ-orbital in the NO+ ion has a pronounced bonding character, then in the CO molecule this orbital is weakly antibonding. Therefore, the CO+ ion has a dissociation energy somewhat higher than the CO molecule. The formation of the other molecules and ions listed above is doubtful, since the energy differences in them are even greater than in CO.
On slides 49, 50, 51 of the presentation LiF, HF molecules are presented, analyze their formation.
The MO LCAO method can be used not only for diatomic molecules, but also for polyatomic ones. As an example, within the framework of this method, we will analyze the structure of the NH 3 ammonia molecule (presentation in Fig. 52).
Since three hydrogen atoms have only three 1 s-orbitals, then the total number of formed molecular orbitals will be equal to six (three bonding and three loosening). Two electrons of the nitrogen atom will be in a non-bonding molecular orbital (lone electron pair of NEP).
metal connection. Unlike ionic and covalent compounds, metals are characterized by high electrical and thermal conductivity. The high electrical conductivity of metals indicates that electrons can freely move throughout its entire volume. In other words, a metal can be considered as a crystal, at the lattice sites of which there are ions bound by electrons that are in common use, i.e., a strongly non-localized chemical bond takes place in metals. The set of electrons that provide this connection is called the electron gas.
A more general approach to the representation of ionic, covalent and metallic crystals can be obtained by applying the representations of the molecular orbital method to them. Let us assume that a solid body is a single molecule formed by a large number of atoms. The outer orbitals of these atoms, when interacting, form bonding and nonbonding molecular orbitals. The energy band formed by bonding molecular orbitals is called valence zone. The zone that unites non-bonding orbitals is called conduction band. The energy differences of electrons within the band are small, and the change in their energy in the band can be represented as a continuous band of energy. There are no energy levels between the conduction band and the valence band. Therefore, electrons cannot be there. The energy band that separates the valence and conduction bands is called forbidden.

Rice. 23. Energy bands in a crystal formed by atomic orbitals.
Electrical conductivity in a solid is ensured by overcoming the band gap by electrons, i.e., the flow of electric current is ensured by the transition of electrons from the valence band to the conduction band. Depending on the band gap, all solids can be divided into three classes: dielectrics, semiconductors, and metal conductors. For insulators, the band gap is more than 3 electron volts; for semiconductors, it ranges from 0.1 to 3 eV. In metals, due to the overlap of the valence band and the conduction band, the band gap is practically absent
When using the method of molecular orbitals, it is considered, in contrast to the method of valence bonds, that each electron is in the field of all nuclei. In this case, the bond is not necessarily formed by a pair of electrons. For example, the H 2 + ion consists of two protons and one electron. Between two protons there are repulsive forces (Fig. 30), between each of the protons and an electron - forces of attraction. A chemical particle is formed only if the mutual repulsion of protons is compensated by their attraction to the electron. This is possible if the electron is located between the nuclei - in the binding region (Fig. 31). Otherwise, the repulsive forces are not compensated by the forces of attraction - they say that the electron is in the region of antibonding, or loosening.
Two-center molecular orbitals
The molecular orbital method uses the idea of a molecular orbital to describe the distribution of electron density in a molecule (similar to the atomic orbital for an atom). Molecular orbitals are the wave functions of an electron in a molecule or other polyatomic chemical particle. Each molecular orbital (MO), like the atomic orbital (AO), can be occupied by one or two electrons. The state of an electron in the binding region is described by the bonding molecular orbital, in the loosening region - by the loosening molecular orbital. The distribution of electrons in molecular orbitals follows the same rules as the distribution of electrons in atomic orbitals in an isolated atom. Molecular orbitals are formed by certain combinations of atomic orbitals. Their number, energy, and shape can be derived from the number, energy, and shape of the orbitals of the atoms that make up the molecule.
In the general case, the wave functions corresponding to molecular orbitals in a diatomic molecule are represented as the sum and difference of the wave functions of atomic orbitals multiplied by some constant coefficients that take into account the proportion of atomic orbitals of each atom in the formation of molecular orbitals (they depend on the electronegativity of atoms):
φ(AB) = s 1 ψ(A) ± s 2 ψ(B)
This method of calculating the one-electron wave function is called "molecular orbitals in the approximation of a linear combination of atomic orbitals" (MO LCAO).
So, when an H 2 + ion or a H 2 hydrogen molecule is formed from two s-orbitals of hydrogen atoms form two molecular orbitals. One of them is binding (it is denoted by σ st), the other is loosening (σ *).
The energies of the bonding orbitals are lower than the energies of the atomic orbitals used to form them. The electrons that populate the bonding molecular orbitals are predominantly located in the space between the bonded atoms, i.e. in the so-called binding region. The energies of the antibonding orbitals are higher than the energies of the initial atomic orbitals. The population of loosening molecular orbitals with electrons contributes to the weakening of the bond: a decrease in its energy and an increase in the distance between atoms in a molecule. The electrons of the hydrogen molecule, which have become common to both bonded atoms, occupy the bonding orbital.

Combination R-orbitals leads to two types of molecular orbitals. Of the two R-orbitals of interacting atoms directed along the bond line, bonding σ St - and loosening σ*-orbitals are formed. Combinations R-orbitals perpendicular to the bond lines give two bonding π- and two loosening π*-orbitals. Using the same rules when populating molecular orbitals with electrons as when filling atomic orbitals in isolated atoms, one can determine the electronic structure of diatomic molecules, for example, O 2 and N 2 (Fig. 35).
From the distribution of electrons in molecular orbitals, the bond order (ω) can be calculated. From the number of electrons located in the bonding orbitals, subtract the number of electrons located in the antibonding orbitals, and the result is divided by 2 n(based on n connections):
ω = / 2 n
It can be seen from the energy diagram that for the H 2 molecule ω = 1.
The molecular orbital method gives the same chemical bond order values as the valence bond method for O 2 (double bond) and N 2 (triple bond) molecules. At the same time, it allows non-integer values of the link order. This is observed, for example, when a two-center bond is formed by one electron (in the H 2 + ion). In this case, ω = 0.5. The magnitude of the bond order directly affects its strength. The higher the bond order, the greater the bond energy and the shorter its length:
Regularities in changes in the order, energy and length of the bond can be traced on the examples of the molecule and molecular ions of oxygen.

The combination of the orbitals of two different atoms with the formation of a molecule is possible only if their energies are close, while the atomic orbitals of an atom of higher electronegativity in the energy diagram are always located lower.

For example, in the formation of a hydrogen fluoride molecule, the combination 1 s-AO of the hydrogen atom and 1 s-AO or 2 s-AO of the fluorine atom, since they differ greatly in energy. Closest in energy 1 s-AO of the hydrogen atom and 2 p-AO of the fluorine atom. The combination of these orbitals causes the appearance of two molecular orbitals: bonding σb and loosening σ*.
Remaining 2 R-orbitals of the fluorine atom cannot be combined with 1 s-AO of the hydrogen atom, since they have different symmetry relative to the internuclear axis. They form non-bonding π 0 -MOs having the same energy as the original 2 R-orbitals of the fluorine atom.
Not participating in LCAO s-orbitals of the fluorine atom form non-bonding σ 0 -MO. Population of nonbonding orbitals by electrons does not promote or prevent bond formation in the molecule. When calculating the link order, their contribution is not taken into account.
Multicenter molecular orbitals

In multicenter molecules, molecular orbitals are multicenter, as they are a linear combination of the orbitals of all the atoms involved in the formation of bonds. In the general case, molecular orbitals are not localized, that is, the electron density corresponding to each orbital is more or less evenly distributed throughout the entire volume of the molecule. However, with the help of mathematical transformations, it is possible to obtain localized molecular orbitals of a certain shape, corresponding to individual two- or three-center bonds or lone electrons.
The simplest example of a three-center bond is the molecular ion H 3 + . Of the three s-orbitals of hydrogen atoms, three molecular orbitals are formed: bonding, non-bonding and loosening. A pair of electrons populates a bonding orbital. The resulting bond is a two-electron three-center bond; the bond order is 0.5.
Chemical particles containing unpaired electrons have paramagnetic properties (in contrast to the diamagnetic properties of chemical particles, in which all electrons are paired). Paramagnets are all substances consisting of chemical particles with an odd number of electrons, such as NO. The method of molecular orbitals makes it possible to identify paramagnets among substances consisting of chemical particles with an even number of electrons, for example, O 2, in the molecule of which two unpaired electrons are located on two loosening π * orbitals.
Chemical species with unpaired electrons in outer orbitals are called free radicals. They are paramagnetic and highly reactive. Inorganic radicals with localized unpaired electrons, for example . H, . NH 2 are usually short lived. They are formed during photolysis, radiolysis, pyrolysis, electrolysis. Low temperatures are used to stabilize them. Short-lived radicals are intermediate species in many reactions.
Fig.1. Contour diagrams of electron density in H 2 +
Lecture No. 4. The concept of the molecular orbital method. Energy diagrams of molecular orbitals for binary homonuclear molecules. σ - and π- molecular orbitals. Dia- and paramagnetic molecules. Ionic bond.
Intermolecular interactions. Hydrogen bond.
The method of valence bonds quite clearly explains the formation and structure of many molecules, but it cannot explain many facts, for example, the existence of molecular ions (H2 +, He2+) or radicals (CH3, NH2), paramagnetism of molecules with an even number of electrons (O2, NO), which are explained within the framework of the molecular orbital method (MMO).
Molecular orbital method
The molecular orbital method, developed by Mulliken and Hund, is based on the assumption that each electron in a molecule is in the field of all the nuclei and electrons of the atoms that form the molecule, and its state is characterized by a wave function Ψ, called the molecular orbital. Each MO corresponds to a wave function that characterizes the region of the most probable stay of electrons of a certain energy in a molecule. Atomic s-, p-, d-, f-orbitals correspond to molecular σ-, π-, δ-, … orbitals, which are filled in accordance with the Pauli principle, Hund's rule, the principle of least energy.
The simplest way to form a molecular orbital (MO) is
linear combination of atomic orbitals (AO) (LCAO-MO method).
If there is one electron in the field of two atomic nuclei A and B, then it can be located either at one nucleus or at another, and its state can be described by two molecular orbitals Ψ and Ψ *, which are formed by a linear combination of atomic orbitals:
Ψ = Ψ A + Ψ B and Ψ * = Ψ A - Ψ B
A molecular orbital is called bonding Ψ if it corresponds to an increase in the electron density in the region between the nuclei and thereby an increase in their attraction, and loosening Ψ * if the electron density decreases between the nuclei and increases behind the nuclei, which is equivalent to an increase in the repulsion of the nuclei. The energy of the binding MO is lower than the energy of the initial AO, the energy of the loosening MO is higher than the energy of the initial atomic orbital.
On fig. 1 shows the contour diagrams of the electron density of the bonding Ψ
(a) and loosening Ψ * (b) molecular orbitals in the H2 + particle.
As in the MVS, the symmetry of molecular orbitals about the bonding line leads to the formation of σ - MO, in the direction perpendicular to the bonding line, - π - MO.
When d-orbitals overlap, δ-
On fig. Figure 2 shows the formation of σ - bonding and σ - loosening MOs with a combination of different atomic orbitals; 3 respectively π -MO and π* - MO.
The overlap of s-orbitals leads to the formation of two molecular orbitals: σs-bonding and σ*s-loosening.
The overlap of p-orbitals leads to the formation of six molecular orbitals of different symmetry. From two p-orbitals of interacting atoms, directed along the communication line, for example, the X axis, a bonding σ p z - and loosening σ * p z -orbitals are formed, along the Z and Y axes - πр z - and πp y - binding and π * р z - and π* p y - loosening MO.

The population of MOs with electrons occurs in accordance with the Pauli principle, the principle of least energy, and Hund's rule.
Rice. 2. Formation of σ - bonding and σ - loosening molecular orbitals
Due to the fact that for orbitals of the same type, the size of the overlapping region of orbitals decreases in the series σ > π > δ , then the splitting of energy levels during the formation of MO from AO decreases in the same order (Fig. 4), which leads to a change in the order of filling σр − and π - MO in molecules.
unpaired electrons with the same spins, for example B, C, N and their electronic counterparts, the sequence of filling MO is as follows:
σ(1s)< σ* (1s) < σ(2s) < σ* (2s) < π (2pz )= π (2py ) < σ(2px ) < π* (2pz )= π* (2py ) < σ* (2px )....

Rice. 3. Formation of π - bonding and π - loosening molecular orbitals
Rice. 4. Reducing the degree of splitting of energy levels in the series σ > π > δ
For homonuclear diatomic molecules of the second and subsequent periods, in which p - sublevels of atoms are filled paired electrons with antiparallel spins, for example (O - Ne) and their electronic counterparts, the sequence of filling MO changes somewhat:
σ(1s)< σ* (1s) < σ(2s) < σ* (2s) < σ(2px ) < π (2pz )= π (2py ) < π* (2pz )= π* (2py ) < σ* (2px )....
The electronic configuration of a molecule can be represented as an energy diagram or an electronic formula.
On fig. Figure 5 shows the energy diagram of molecular orbitals for the hydrogen molecule H2, the electronic formula of which is written as follows: [σ(1s)]2 or (σ 1s )2.

Rice. 5. Energy diagram of the H 2 molecule
The filling of the bonding molecular orbital σ 1s leads to an increase in the electron density between the nuclei and determines the existence of the H2 molecule.
The MO method substantiates the possibility of the existence of the molecular hydrogen ion H2 + and the impossibility of the existence of the He2 molecule, since in the latter case, the filling of the bonding and loosening σ 1s orbitals with two electrons does not lead to a change in the energy of isolated atoms: [(σ 1s )2 (σ * 1s )2 ] (Fig. 6). Therefore, the He2 molecule does not exist.
Rice. 6. Energy diagram confirming the impossibility of the existence of the He2 molecule
On fig. Figure 7 shows the energy diagram of molecular orbitals formed by the overlap of s- and p-orbitals of the second energy level for diatomic homonuclear molecules of the A2 type.
The arrows show the change in the order of occupation of the MO of molecules formed by atoms, in which the 2p sublevel is filled with unpaired electrons (B2, C2, N2), for which the binding π st (2py ) and π st (2pz ) are located below σst (2px ), and paired electrons (O2 , F2 , Ne2 ), for which the bonding π st (2py ) and π st (2pz ) are located above σst (2px ),

Rice. Fig. 7. MO energy diagram for homonuclear molecules of the 2nd period (arrows show the change in the filling order of the bonding σ- and π-MO)
In MMO, the concept is used - bond order, which is defined as the difference between the number of electrons on the bonding MO and the number of electrons on the loosening MO, divided by the number of atoms that form the bond.
N − N* |
||||||||
For diatomic molecules, the bond order n is: n = |
Where N is the number |
|||||||
electrons on bonding MOs, N* is the number of electrons on loosening MOs. |
||||||||
For the H2 molecule, the bond order is, respectively, |
2− 0 |
1 , for He2 |
||||||
2− 2 |
Which confirms the impossibility of the existence of a diatomic |
|||||||
molecules. It is known that inert gases exist in the form of monatomic molecules. Using the same rules for populating molecular orbitals with electrons as
when filling atomic orbitals in isolated atoms (Pauli principle, minimum energy principle and Hund's rule)), one can determine the electronic structure of diatomic molecules, for example N2 and O2.
Let us write the electronic configurations of atoms in the ground state:
or . |
|||
or . |
|||
The electronic configurations of N2 and O2 molecules can be written as follows |
|||
N + N → N2 |
|||
O2 : O+O → O2

On fig. 8 shows the energy diagram of the formation of an oxygen molecule.
Fig.8. Energy diagram of an oxygen molecule
In the O2 molecule, two electrons with parallel spins ended up on two
degenerate (with the same energy) * -loosening molecular orbitals. The presence of unpaired electrons determines the paramagnetic properties of the oxygen molecule, which become especially noticeable if oxygen is cooled to a liquid state.
Molecules of paramagnets have their own magnetic moment due to the internal movement of charges. In the absence of an external magnetic field, the magnetic moments of the molecules are randomly oriented, so the resulting magnetic field due to them is zero. The total magnetic moment of the substance is also equal to zero.
If the substance is placed in an external magnetic field, then under its influence the magnetic moments of the molecules acquire a predominant orientation in one direction, and the substance becomes magnetized - its total magnetic moment becomes different from zero.
Molecules of diamagnets do not have their own magnetic moments and are weakly magnetized when introduced into a magnetic field.
Paramagnets are all substances consisting of chemical particles with an odd number of electrons, for example, the NO molecule, molecular ions N2 +, N2 -, etc.
Most substances whose molecules contain an even number of electrons have diamagnetic properties(N2, CO).
An explanation of the paramagnetic properties of oxygen and boron molecules containing an even number of electrons is given on the basis of MMO. The O2 molecule has two unpaired electrons in the *-loosening molecular orbitals, and the B2 molecule has two unpaired electrons in the *-bonding molecular orbitals (see Table 1).
Chemical particles that have unpaired electrons in their outer orbitals are called free radicals. They are paramagnetic and highly reactive. Inorganic radicals with localized unpaired electrons, for example (.H), (.NH2), are usually short-lived. They are formed during photolysis,

radiolysis, pyrolysis, electrolysis. Low temperatures are used to stabilize them. Short-lived radicals are intermediate particles in many reactions, especially chain and catalytic ones.
The bond order in the N2 molecule, which has an excess of six electrons per
The concept of the order of a chemical bond in the MO method coincides with the concept of the multiplicity of bonds in the BC method (O2 is a double bond, N2 is a triple bond). The magnitude of the bond order affects the strength of the bond. The higher the bond order, the greater the bond energy and the shorter the bond length.
In table. 1 shows the electronic configurations and bond characteristics for homonuclear molecules of the first and second periods. As can be seen from the table, with an increase in the bond order in the series B2 - C2 - N2, the energy increases and the bond length decreases.
Table 1. Electronic configurations and some properties of molecules of the first and second periods
Magnetic |
|||||||
Molecule |
Electronic configuration |
disconnection, |
|||||
properties |
|||||||
[(σ1s )2 ] |
diamagnetic |
||||||
[(σ1s )2 (σ*1s )2 ] |
Molecule does not exist |
||||||
diamagnetic |
|||||||
Molecule does not exist |
|||||||
paramagnetic |
|||||||
diamagnetic |
|||||||
diamagnetic |
|||||||
The MO method allows non-integer values of the link order. This takes place in molecular ions, for example, in the molecular ion H2+, for which n = 0.5.
Regularities in changes in the order, energy and length of the bond can be traced on the examples of the molecule and molecular ions of oxygen.
The electronic configuration and bond order of the oxygen molecule are given in Table. 1. Electronic configurations and bond order of molecular oxygen ions
the following: |
|||||||
O2 - - |
n = 1.5. |
||||||
The decrease in the bond order in the series of particles O2 + , O2 , O2 - determines the decrease |
|||||||
bond strength and finds experimental confirmation: |
|||||||
O2+ : |
n \u003d 2.5, E sv \u003d 629 kJ / mol, |
d sv = 112 pm; |
|||||
n \u003d 2.0, E sv \u003d 494 kJ / mol, |
d sv = 121 pm; |
||||||
O2 - : |
n \u003d 1.5, E sv \u003d 397 kJ / mol, |
d sv \u003d 126 pm. |
|||||
All particles have unpaired electrons and exhibit paramagnetic properties. Molecules that have the same number of valence electrons are called
isoelectronic particles. These include CO and N2 molecules, which have a total of 14 electrons; molecular ion N2 + and molecule CN, having 13 electrons. IMO assigns the same filling order to isoelectronic particles

electrons of molecular orbitals, the same bond order, which makes it possible to explain the closeness of the physical properties of molecules.
When a heteronuclear molecule of type AB is formed, the combination of orbitals of two different atoms, leading to the formation of a molecule, is possible only if the electron energies are close, while the orbitals of an atom with a higher electronegativity in the energy diagram are always located lower.
On fig. Figure 9 shows the energy scheme for the formation of a CO molecule.
Four 2p electrons of the oxygen atom and two 2p electrons of the carbon atom pass to the binding π - and σ - MO. The energy of the 2p electrons of the connecting atoms is not the same: the oxygen atom has a higher nuclear charge and electronegativity compared to the carbon atom, therefore the 2p electrons in the oxygen atom are more strongly attracted by the nucleus and their position on the energy diagram corresponds to a lower energy compared to the 2p orbitals of the carbon atom . All six electrons involved in bond formation are located on three bonding MOs; therefore, the bond multiplicity is three, which explains the significant similarity in the properties of free nitrogen and carbon monoxide (II) (Table 2).
Rice. 9. Energy scheme for the formation of the CO molecule
Table 2. Some physical properties of CO and N2 molecules
Molecule |
T pl , K |
T bale, K |
E St, kJ/mol |
d sv , pm |
Non-valent types of chemical bond
Ionic bond.
When the difference in the electronegativity of the interacting atoms is more than two units, the displacement of valence electrons is so large that we can talk about their transition from one atom to another with the formation of charged particles - cations and anions. These particles interact with each other according to the laws of electrostatics. The resulting bond is called ionic. Compounds with ionic bonds are significantly
less common than compounds with a covalent bond, characteristic of substances that exist under normal conditions in the crystalline state and have ionic conductivity in the molten or dissolved state. Ionic compounds primarily include typical salts - alkali metal halides having an ionic crystal lattice. Ionic molecules exist only at high temperatures in vapors of ionic compounds.
The ionic bond, unlike the covalent bond, is non-directional, since the ions form spherically symmetrical force fields, does not have saturation, since the interaction of ions of the opposite sign occurs in different directions, is delocalized, since no increased electron density is observed in the binding region.
Electrostatic model of ionic bond considers its formation as the interaction of oppositely charged ions, each of which is characterized
The formation energy of an AB molecule can be defined as the algebraic sum of several energies: the attraction energy of Az+ and Bz- ions, the repulsion energy of ions, the electron affinity energy of atom B, and the ionization energy of atom A.
ions in a molecule, n - takes into account the share of the repulsion energy, which is usually 10% of the attraction energy, E B - the energy of the electron affinity of the atom B, I A - the ionization energy of the atom A.
For a gaseous KCl molecule, the energy E AB was calculated without taking into account the polarization
ions: d \u003d 2.67 10-10 eV, E Cl \u003d 3.61 eV, I K \u003d 4.34 eV and the binding energy is E bond \u003d -E AB \u003d 4.06 eV ~ 391 kJ ..
The experimentally determined ionization energy of the KCl molecule is 422 kJ/mol.
In gases, liquids and crystals, each ion tends to surround itself with the largest number of ions of opposite charge.
The location of ions in space is determined by the ratio of their radii. If the ratio of the cation radius to the anion radius is within
r + /r - = 0.41-0.73, then six ions of opposite charge are coordinated around the central atom - a cation or anion. This coordination is called octahedral, and the type of crystal lattice is designated as the NaCl type.
If the ratio of the cation radius to the anion radius is within
r + /r - = 0.73-1.37, then eight ions of opposite charge are coordinated around the central atom - a cation or anion. Such coordination is called cubic, and the type of crystal lattice is designated as the CsCl type.
When ions approach each other, their spherical electron shells are deformed, which leads to a displacement of the electric charge and the appearance of an induced electric moment in the particle. This phenomenon is called ion polarization. Ion polarization is a two-way process that combines the polarizability of ions and polarizing effect depending on the electronic structure, charge, and size of the ion. Polarizability is minimal for ions with an inert gas configuration (ns 2 np 6 ), which at the same time have the greatest polarizing effect. Significant polarizability of ions of d - elements is explained by the presence of a large number of valence electrons, as a result, the covalent component of the bond increases.
The polarization effect explains many differences in the properties of substances, for example, the poor solubility of silver chloride in water compared to alkali chlorides.

metals, differences in melting temperatures, for example, T pl, AgCl = 4550 C, T pl, NaCl = 8010 C. Electronic configurations of ions: Ag + - 4d 10 5s 0; Na+ - 3s 0 .
The less symmetric electronic configuration of the Ag+ ion due to the presence of 4d 10 electrons causes its stronger polarization, which leads to the appearance
directional covalent component of the bond compared to NaCl, in which the degree of ionicity of the bond is higher.
Metal connection.
The most important property of metals is high electrical conductivity, which decreases with increasing temperature. Metal atoms differ from atoms of other elements in that they retain their outer electrons relatively weakly. Therefore, in the crystal lattice of a metal, these electrons leave their atoms, turning them into positively charged ions. "Shared" electrons move in space between cations and keep them together. Interatomic distances in metals are greater than in their compounds with a covalent bond. Such a bond exists not only in metal crystals, but also in their melts and in the amorphous state. It is called
metallic, determines the electronic conductivity of metals.
Electrons in a metal move randomly, passing from one atom to another, forming an electron gas. Positively charged metal ions only slightly oscillate around their position in the crystal lattice, when the metal is heated, the vibrations of the cations increase and the electrical resistance of the metal increases. Due to the presence of free electrons not associated with certain atoms, metals conduct electricity and heat well.
Such physical properties of metals as high thermal and electrical conductivity, ductility and ductility, metallic luster can be explained based on the concept of electron gas. The metallic bond is quite strong, since most metals have a high melting point.
A more rigorous interpretation of the metallic bond allows us to give molecular orbital method. Recall that when two atomic orbitals interact, two molecular orbitals are formed: a bonding and an antibonding orbital. There is a splitting of the energy level into two. If four metal atoms interact simultaneously, four molecular orbitals are formed. With the simultaneous interaction of N particles contained in a crystal, N molecular orbitals are formed, and the value of N can reach huge values comparable to the number
Avogadro (6 1023 ). Molecular orbitals formed by atomic orbitals of the same sublevel are so close that they practically merge, forming a certain
energy zone (Fig. 10).
Rice. 10. Formation of an energy band in a crystal
Consider the formation of energy bands on the example of metallic sodium,
Working programm.Method of molecular orbitals. Molecular orbital as a linear combination of atomic orbitals. The concept of bonding and loosening molecular orbitals. Communication order. The sequence of increasing the energy of the molecular orbitals of the elements of the 1st and 2nd periods of the PSEM. Electronic formulas of molecules. Principles of filling molecular orbitals. Molecular diagrams of diatomic homo- and heteronuclear molecules. Magnetic properties of molecules (diamagnetism and paramagnetism).
The method of valence bonds makes it possible in many cases to explain the formation of a chemical bond and to predict a number of properties of molecules. Nevertheless, many compounds are known whose existence and properties cannot be explained from the standpoint of the VS method. More versatile is molecular orbital method (MO).
The VS method is based on the idea of the formation of a chemical bond by a pair of electrons belonging to two atoms. According to the MO method, the electrons that form a chemical bond move in the field formed by the nuclei of all the atoms that make up the molecule, i.e. electrons belong to all atoms of the molecule. Therefore, molecular orbitals are generally multicenter.
According to the MO method, all the electrons of a given molecule involved in the formation of a chemical bond are distributed over the corresponding molecular orbitals. Each molecular orbital, like the atomic orbital, is characterized by its own set of quantum numbers.
Molecular orbitals are obtained by adding or subtracting the original atomic orbitals. If MO is formed from atomic orbitals ψ A and ψ B, then when they are added, MO ψ + arises, and when subtracted - ψ -:
ψ + = c 1 ψ A + c 2 ψ B,
ψ - = s 3 ψ A - s 4 ψ B,
where c 1 – c 4 are the coefficients that determine the share of participation of the corresponding atomic orbital in MO.
This operation is called linear combination of atomic orbitals, so the method is called MO LCAO(a molecular orbital is a linear combination of atomic orbitals). The number of formed MOs is equal to the number of initial AOs. Molecular orbitals are formed only from atomic orbitals with similar energies. Large differences in the energies of the initial AO prevent the formation of MOs. Orbitals of internal energy levels do not participate in the formation of MO.
When adding AO, they form binding MO with an energy lower than that of the original AO. Subtraction of AO leads to the formation loosening MO, which have a higher energy compared to the original AO. The scheme for the formation of bonding and loosening MOs from 1s atomic orbitals is shown in Fig. . 6.11.
Electrons located on the MO are characterized by four quantum numbers
n is the main quantum number;
l is the orbital quantum number;
Rice. 6.11. Scheme of formation of bonding (σ1s) and loosening (σ*1s) molecular orbitals
λ is the molecular quantum number similar to the magnetic quantum number m l ; can take values 0; ±1; ±2, denoted by the letters σ, π, δ, respectively;
m s is the spin quantum number.
The filling of molecular orbitals with electrons obeys the Pauli principle, the principle of least energy, and Hund's rule.
The sequence of increasing MO energies, i.e. the filling sequence, for the elements of the beginning of the 2nd period (for nitrogen inclusive) has the form
σ1s<σ*1s<σ2s<σ*2s<π2p х =π2p z <σ2p y < π*2p х =π*2p z <σ*2p y ,
and for the elements of the end of the 2nd period (O, F, Ne) -
σ1s<σ*1s<σ2s<σ*2s< σ2p y <π2p х =π2p z < π*2p х =π*2p z <σ*2p y .
The half-difference in the number of electrons in bonding (N sv) and loosening (N p) orbitals is called order(multiplicity) connections n:
A molecule is formed if n>0, i.e. a bond can be formed not only by a pair, but also by one electron, and, therefore, the bond order can be not only an integer, but also a fractional number. As the order increases, the binding energy increases.
Diatomic homonuclear molecules of elements of the 1st period. The simplest molecule is the molecular ion. In accordance with the principle of least energy, the only electron of the molecule is located on σ1s MO. Therefore, the electronic formula of a molecular ion will be written as
An electronic formula can be represented graphically as molecular (energy) diagram(Fig. 6.12), showing the relative energies of atomic and molecular diagrams and the number of electrons on them.
The bond order of the ion is n=(1-0)/2=0.5, therefore, this particle can exist.
|



Rice. 6.12. Molecular diagram
A molecular ion has one electron, so it is paramagnetic, i.e. is drawn into the magnetic field. All substances with unpaired electrons are paramagnetic.
The hydrogen molecule H 2 contains two electrons, and its electronic formula is as follows:
Н 2 [(σ1s) 2 ].
From the molecular diagram (Fig. 6.13) it follows that the bond order of the hydrogen molecule is equal to one. An increase in the bond order from 0.5 to 1 upon passing from to H 2 is accompanied by an increase in the binding energy from 236 to 436 kJ/mol and a decrease in the bond length from 0.106 to 0.074 nm.
The electrons of the H 2 molecule are paired, and for this reason molecular hydrogen diamagnetic, i.e. pushed out of the magnetic field. Diamagnets are all substances that do not contain unpaired electrons.

Fig.6.13. Molecular diagram of H 2
The second element of the 1st period, helium, in accordance with the ideas of the MO method, can form a paramagnetic molecular ion (n = 0.5), and the He 2 molecule cannot exist, because the number of electrons in the bonding and loosening molecular orbitals is the same and the bond order is zero.
Diatomic homonuclear molecules of elements of the 2nd period. Consider, as an example, an oxygen molecule. The twelve electrons of the outer levels of two oxygen atoms (2s 2 2p 4) will fill the molecular orbitals as follows:
About 2 .
The symbol K in the electronic formula means that the electrons of the K-level (1s 2) do not participate in the formation of molecular orbitals. The molecular diagram of the oxygen molecule is shown in fig. 6.14. In accordance with Hund's rule, two electrons in the orbitals π2p x and π2p z are unpaired and the oxygen molecule is paramagnetic, which is confirmed experimentally. Note that it is impossible to explain the paramagnetism of oxygen within the framework of the valence bond method. The bond order in the O 2 molecule is n=(8-4)/2=2.

Rice. 6.14. Molecular diagram of O 2
The bond order in diatomic homonuclear molecules of elements of the 2nd period increases from 1 for B 2 to 3 for N 2, and then decreases to 1 for F 2. The formation of Be 2 and Ne 2 molecules is impossible, because the bond order in these molecules is zero.
Diatomic heteronuclear molecules of elements of the 2nd period. The atomic orbitals of different atoms contribute differently to the molecular orbitals, or equivalently, the coefficients with i in the equations
ψ + = c 1 ψ A + c 2 ψ B;
ψ - = s 3 ψ A - s 4 ψ B
are not equal to one. The atomic orbital of the more electronegative element contributes more to the bonding orbital, and the AO of the more electropositive element contributes more to the antibonding orbital. If atom B is more electronegative than atom A, then c 2 > c 1, and c 3 > c 4. The binding MOs are closer in energy to the AOs of a more electronegative atom, while the loosening MOs are closer to the AOs of a more electropositive atom.
As an example, consider the CO molecule. Ten electrons of carbon and oxygen atoms will be placed in the MO as follows:
CO .
The bond order in the CO molecule is n=(8-2)/2=3. The CO molecule is paramagnetic. The molecular diagram is shown in fig. 6.15.

Rice. 6.15. Molecular diagram of CO
metal connection
Working programm.Metal connection. Energy band, valence band, conduction band, forbidden band. Conductors, semiconductors, insulators.
Metals that make up most of the D.I. Mendeleev, have a number of features:
1) metallic luster, i.e. high reflectivity to light;
2) high thermal and electrical conductivities;
3) plasticity and malleability.
These properties of metals are explained by a special type of covalent bond called metallic bond.
From the standpoint of the molecular orbital method, a metal crystal is one huge molecule. The atomic orbitals of an atom overlap with the atomic orbitals of neighboring atoms, forming bonding and loosening MOs. These
the orbitals overlap, in turn, with the atomic orbitals of the next neighbors, and so on.
As a result, the atomic orbitals of all the atoms that make up the metal crystal overlap, and a huge number of MOs are formed that spread over the entire crystal (Fig. 6.16).
Metals have high coordination numbers, usually 8 or 12, i.e. each atom is surrounded by 8 or 12 neighbors. For example, the coordination number of lithium is 8. Therefore, the 2s-atomic orbital of lithium overlaps with the 2s-atomic orbitals of eight neighboring atoms, which, in turn, overlap with the atomic orbitals of their neighbors, and so on. In 1 mole
overlap occurs 6.02 . 10 23 atomic orbitals to form the same number of molecular orbitals. The difference in the energies of these orbitals is very small and amounts to about 10 -22 eV (10 -21 kJ). Molecular orbitals form energy zone. The filling of the energy band with electrons occurs in accordance with the rules
least energy, Hund's rule and Pauli's exclusion. Consequently, the maximum number of electrons in the energy band formed by s-electrons will be 2N, where N is the number of atoms in the crystal. Accordingly, up to 6N, 10N or 14N electrons can be located in the zones formed by p-, d- and f-orbitals.
 |
Rice. 6.16. Scheme of the formation of the energy zone
A zone filled with electrons that carry out a chemical bond is called valence band. This zone can be filled to varying degrees, depending on the nature of the metal, its structure, etc. Above the valence band is a free band called conduction band. Depending on the nature of the atoms and the structure of the crystal lattice, the valence and conduction bands may overlap or be separated by an energy gap called forbidden zone. If the valence and conduction bands overlap, the substances are classified as metals. If the band gap is ΔЕ=0.1÷3.0 eV, then the substances are semiconductors, if ΔЕ>3 eV, then they are insulators.
The valence band of metals is usually incompletely filled with electrons. Therefore, the transfer of electrons to the conduction band requires very little energy, which explains the high electrical and thermal conductivity of metals.
intermolecular bond
Working programm.Intermolecular bond. Van der Waals forces: orientational, induction, dispersion. Hydrogen bond. Influence on the physico-chemical properties of substances.
Attractive forces always act between electrically neutral atoms and molecules in solid, liquid and gaseous states. This is evidenced, for example, by the non-ideality of real gases, the decrease in the temperature of gases during expansion, the existence of noble gases in a condensed state, etc.
There are two types of intermolecular interactions:
1) Van der Waals forces;
2) hydrogen bond.
Sometimes intermolecular interactions include donor-acceptor and metallic bonds.
Van der Waals forces. The main properties of van der Waals forces are low energy (up to ~40 kJ/mol) and unsaturation. There are three types of van der Waals forces: orientation, induction and dispersion.
Orientational (dipole-dipole) interaction occurs only between polar molecules. At sufficiently small distances between molecules, the oppositely charged ends of the dipoles attract, and the like-charged ends repel (Fig. 6.17, a) The larger the dipole moments of the molecules, the stronger the orientational interaction. Orientational interaction weakens with increasing temperature and distance between molecules.
Inductive interaction occurs between molecules of different polarity. Under the action of an electric field of a more polar molecule, a non-polar or low-polarity molecule is polarized, i.e. a dipole arises (is induced) in it or the dipole moment increases (Fig. 6.17, b). The energy of the inductive interaction is determined by the value of the dipole moment of the polar molecule, the distance between the molecules, and polarizability non-polar molecule, i.e. its ability to form a dipole under the action of an external field.
Dispersion interaction is the most universal, i.e. acts between any molecules regardless of their polarity. The nucleus of an atom and an electron form instantaneous dipoles, which induce instantaneous dipoles in neighboring particles (Fig. 6.17, c). The synchronous motion of instantaneous dipoles of different molecules leads to a decrease in the energy of the system and the attraction of particles. The dispersion interaction energy increases with increasing polarizability of particles and decreasing distance between them and does not depend on temperature.

Rice. 6.17. Van der Waals interactions: a – orientational; b - induction; c - dispersive
The energy of van der Waals interactions is inversely proportional to the sixth power of the distance between the centers of the interacting particles. With a strong approach of molecules, repulsive forces between the electron shells begin to act, which balance the forces of attraction.
The relative values of various types of van der Waals interaction for some substances are given in Table. 6.1.
Table 6.1. Contribution of individual components to the van der Waals interaction energy
From Table. 6.1 it follows that an increase in the dipole moment leads to an increase in the orientational and induction interactions, and an increase in the polarizability is accompanied by an increase in the dispersion interaction.
hydrogen bond is a special type of intermolecular interaction that takes place between the molecules of compounds containing F-H, O-H, N-H groups, i.e. a hydrogen atom and an element with a very high electronegativity.
The electronic density of the E-N bond is shifted towards the electronegative element. The hydrogen atom loses its electron shell and becomes a proton. Due to its small size and the absence of repulsion of electron shells, the proton is able to enter into an electrostatic interaction with the electron shell of a strongly electronegative atom of a neighboring molecule. At the same time, hydrogen acts as an acceptor of an electron pair provided by an electronegative atom of a neighboring molecule.
![]() .
.
The van der Waals and repulsive forces also contribute to the formation of the hydrogen bond.
Unlike van der Waals forces, the hydrogen bond has the properties of directionality and saturation.
The hydrogen bond energy is low, ranging from 8 to 40 kJ/mol, and increases in the series N-H< O-H < F-H. Тем не менее наличие водородной связи оказывает сильное влияние на физико-химические свойства веществ. Так, молекула воды может участвовать в образовании четырех водородных связей. Это ведет к образованию прочных ассоциатов (Н 2 О) n , что объясняет высокую температуру плавления и кипения воды по сравнению с ее аналогом – H 2 S (t кип =-61,8 о С), высокую теплоёмкость (4,218 кДж/кг К при температуре 273К), высокую энтальпию испарения (2250 кДж/кг). Сероводород как соединение с более высокой молекулярной массой должен был бы иметь более высокую температуру кипения, чем вода. Отсутствие сильных водородных связей у H 2 S приводит к обратной зависимости.
The hydrogen bond explains the formation of hydrogen fluoride (HF) n associates, the dimerization of carboxylic acids:
![]()
Many chemical compounds contain N-H and O-H chemical bonds, hence hydrogen bonds are very common. Hydrogen bonds play a particularly important role for biological objects. Thus, the double helixes of DNA are connected by intermolecular hydrogen bonds.
Questions for self-study
1. Consider the formation of a covalent bond using the example of a hydrogen molecule. Give a graph of the dependence of the potential energy of a system of two hydrogen atoms on the internuclear distance.
2. How to explain the greater stability of the F 2 molecule compared to a system of two free fluorine atoms?
3. Give the electronic structures of the atoms of the elements of the 2nd period in the ground and excited states.
4. Why does the argon atom not form chemical bonds?
5. Why does an oxygen atom form 2 chemical bonds, and a sulfur atom - 6?
6. Hybridization of atomic orbitals. Factors favoring hybridization. Orientation of hybrid orbitals in space. sp-, sp 2 -, sp 3 - and sp 3 d 2 - hybridization.
7. Give examples of the influence of non-bonding (lone) electron pairs on the stereochemistry of molecules.
8. How does the position of an element in the PSEM affect the stability of hybridization of atomic orbitals? Give examples.
9. Determine the type of hybridization of the orbitals of the central atom in the hydronium ion H 3 O + . Draw the geometric shape of this particle.
10. What are the features of the donor-acceptor mechanism for the formation of a covalent bond? Give examples of particles capable of playing the role of an electron pair donor and acceptor.
11. What factors affect the energy of a chemical bond?
12. Arrange the following compounds in order of increasing bond length: NaH, NaF, NaCl, NaBr.
13. Arrange the following bonds in order of increasing their energy: a) O-O; O=O; b) O-O; S-S; c) H-F; H-Cl; H-Br; d) Li-H; Be-H; B-H; C-H.
14. Which of the bonds is stronger: a) C-F or C-Br; b) C=O or C-O; c) O=O or S=S?
15. In which of the following compounds is the bond of the central atom saturated: a) IF 3 ; IF 5 ; IF7; b) Cl 2 O; ClO 2 ; Cl2O7?
16. Determine the coordination number of the aluminum atom in the compounds: a) Li; b) Na 3 .
17. Arrange the following chemical bonds in order of increasing polarity: Na-O; Na-F; Na-N.
18. Which of the HF, HCl, HBr, HI molecules has the longest dipole?
19. How does the polarity of a bond change in the series HF, HCl, HBr, HI?
20. What factors affect the magnitude of the dipole moment of the following molecules: a) NH 3, b) PH 3, c) AsH 3? Which of these molecules can have the largest dipole moment?
21. What type of bond is realized in the following molecules: HCl, Cl 2 , RbCl, ClF?
22. Arrange the following ions in decreasing order of their polarizing power: Na + ; Mg2+; Al3+.
23. Arrange the following ions in order of increasing polarizability: F - ; Cl-; Br - ;I - .
24. In an aqueous solution of which salt, the O-H bond in a water molecule will be polarized to a greater extent: NaCl; MgCl 2 ; AlCl 3 ? Why?
25. What is the reason for the increase in the strength of hydrohalic acids, observed with an increase in the serial number of the halogen atom?
26. What is the reason for the increase in the strength of hydroxides of alkaline and alkaline earth elements, observed with an increase in the charge of the nucleus of metal atoms?
27. Why is sulfuric acid stronger than sulfuric acid?
28. Why is acetic acid CH 3 COOH much weaker than trifluoroacetic acid CF 3 COOH?
29. Determine how many σ- and π-bonds does a molecule of butadiene CH 2 CHCHCH 2 contain?
Answer: 9 σ- and 2 π-bonds.
30. Determine how many σ- and π-bonds does a vinylacetylene molecule CHCCHCH 2 contain?
Answer: 7 σ- and 3 π-bonds.
31. State the main provisions of the molecular orbital method.
32. What is the main difference between the MO method and the VS method?
33. Make an electronic formula and give a molecular diagram of the nitrogen molecule. Determine the bond order and indicate the magnetic characteristics of the molecule.
34. Make an electronic formula and give a molecular diagram of the fluorine molecule. Determine the bond order and indicate the magnetic characteristics of the molecule.
35. Make an electronic formula and give a molecular diagram of a molecule of nitric oxide (II). Determine the bond order and indicate the magnetic characteristics of the molecule.
36. Explain from the standpoint of the molecular orbital method the increase in binding energy in the series fluorine, oxygen, nitrogen.
37. How will the chemical bond energy change during the transition from F 2 to and?
38. Which of the following molecules should not exist: a) C 2, b) Li 2, c) Be 2, d) B 2?
39. What physical properties are characteristic of metals?
40. Describe the features of the chemical bond in metals and its characteristics.
41. What are the reasons for the difference in the electrical conductivity of metals, semiconductors and insulators?
42. Give examples of physical phenomena that indicate the presence of interactions between neutral atoms and molecules.
43. Describe the mechanism of occurrence and characteristics of van der Waals interactions.
44. What types of van der Waals interactions can take place for the following substances: helium, methane, nitrogen, hydrogen bromide?
45. What type of van der Waals forces prevails in each of the following substances: O 2 , H 2 O, OF 2 ?
46. Describe the interactions that contribute to the formation of a hydrogen bond.
47. What type of bond is realized during the formation of an H 3 O + ion from a proton and a water molecule?
48. For which of the following compounds are hydrogen bonds possible: SiH 4 , HCOOH, CH 3 CH(NH 2)COOH, H 2 O 2 , HCl?
49. Why is the boiling point of ammonia NH 3 higher than that of phosphine PH 3?
50. Why can monobasic hydrofluoric acid form acidic salts, such as NaHF 2 , but hydrochloric acid does not form similar compounds?
6.6. Tasks for current and intermediate controls
1. Explain the term "atomic orbital overlap".
2. Can we say that the noble gases He, Ne and others consist of molecules?
3. Why is hydrochloric acid stronger than hydrofluoric acid?
4. What is the reason for the formation of any chemical bond? What energy effect is accompanied by this process?
5. How does the bond strength change in the series HF, HCl, HBr, HI? State the reasons for these changes.
6. Predict which of the bonds is stronger: a) C - F or C - Br; b) C \u003d O or C - O; c) O - O or S - S.
7. Arrange the following bonds in order of increasing polarity: Na - O, Na - F, Na - N.
8. Arrange these bonds in ascending order of polarity: a) H - F, H - C, H - H; b) P - S, Si - Cl, Al - Cl.
9. What is the name of the distance between the centers of the nuclei of atoms in a molecule and how does it affect the strength of chemical bonds?
10. Why and how does the size of atoms affect the length and energy of the bond formed between them?
11. What explains the greater stability of a system of two bound atoms (for example, H 2) compared to a system of two free atoms (2H)?
12. What explains the ability of atoms of many elements to form a number of bonds that exceed the number of unpaired electrons in their atoms in the ground state?
13. Indicate the factors contributing to the hybridization of atomic orbitals.
14. Explain how a carbon atom with two unpaired electrons can show a covalence of four?
15. Compare the mechanism for the formation of covalent bonds in CH 4, NH 3 molecules and in the ion.
16. Give the scheme of overlapping atomic orbitals in BeCl 2 and BF 3 molecules.
17. Which of the HF, HCl, HBr or HI molecules has the longest dipole length?
18. Arrange the indicated bonds in order of increasing polarity:
a) H - F, F - C, F - F;
b) C – N, B – O, Li – l;
c) P - S, Si - Cl, Al - Cl
19. Arrange in ascending order the degree of ionicity of the B - Cl, Na - Cl, Ca - Cl, Be - Cl bonds.
20. For which bonds the dipole length a) is equal to zero; b) less than the length of bonds; c) equal to the length of bonds?
21. Specify the donor and acceptor in the reaction H 2 O + H + = H 3 O + .
22. Which of the following molecules should have the largest dipole moment: NH 3 , PH 3 , AsH 3 , BH 3 ?
23. What bond is called hydrogen? How does it affect the physical properties of substances?
24. Consider a particle from the point of view of the MO method. Can this particle exist? What is the bond order and magnetic properties of this particle?
25. Consider a particle from the point of view of the MO method. Can this particle exist? What is the bond order and magnetic properties of this particle?
26. Consider a particle from the point of view of the MO method. Can this particle exist? What is the bond order and magnetic properties of this particle?
27. Consider a particle from the point of view of the MO method. Can this particle exist? What is the bond order and magnetic properties of this particle?
28. Consider a particle from the point of view of the MO method. Can this particle exist? What is the bond order and magnetic properties of this particle?
29. Consider a molecule from the standpoint of the MO method. What is the bond order and magnetic properties of this molecule?
30. Consider a molecule from the standpoint of the MO method. What is the bond order and magnetic properties of this molecule?
Bibliographic list
1.Pirogov, A.I. General chemistry: textbook. allowance / A.I. Pirogov; Ivan. state energy un-t. – Ivanovo, 2010. – 220 s.
2.Pirogov, A.I., General chemistry: textbook.-method. programmed manual / A.I. Pirogov, A.V. Ionov; Ivan. state energy un-t. – Ivanovo, 2012. – 76 p.
3.Plastic bag assignments for current and intermediate controls: method. development for first-year students / I.M. Arefiev [and others]; ed. A.I. Pirogov; Ivan. state energy un-t. - Ivanovo, 2011. - 72 p.
4.methodical instructions for performing laboratory work in general chemistry / V.K. Abrosimov [and others]; ed. VC. Abrosimov; Ivan. state energy un-t. - Ivanovo, 2000. - 44 p.
5.Korovin, N.V. General chemistry (bachelor's degree) / N.V. Korovin. – 13th ed. – M.: Academy, 2011. – 496 p.
6. Tasks and exercises in general chemistry: textbook. allowance / B.I. Adamson [and others]; ed. N.V. Korovin. - 3rd ed. - M .: Higher. school, 2006. - 255 p.
7.Korovin, N.V. Laboratory work in chemistry: textbook. allowance for universities / N. V. Korovin [and others] - 4th ed. - M .: Higher. school, 2007 - 256 p.
8.Glinka, N.L. General chemistry: textbook. for bachelors / N.L. Glinka; under. ed. V.A. Popkova, A.V. Babkov. - 19th ed., revised. and additional – M.: Yurayt, 2014. – 900 p. - (Series "Bachelor. Basic course").
9.Glinka, N.L. Tasks and exercises in general chemistry: studies.-pract. manual for bachelors / N.L. Glinka; under. ed. V.A. Popkova, A.V. Babkov. – 14th ed. – M.: Yurayt, 2014. – 236 p. - (Series "Bachelor. Basic course").
10.Glinka, N.L. Workshop on General Chemistry: Proc. allowance for academic. undergraduate / N.L. Glinka; under. ed. V.A. Popkova, A.V. Babkova, O.V. Nesterova. – M.: Yurayt, 2014. – 248 p. - (Series "Bachelor. Academic course").
11.Stepin, B.D. Application of the international system of units of physical quantities in chemistry / B.D. Stepin. - M .: Higher. school, 1990. - 96 p.
3.4. Molecular orbital method
The molecular orbital (MO) method is most visible in its graphical model of a linear combination of atomic orbitals (LCAO). The MO LCAO method is based on the following rules.
1. When atoms approach each other to the distances of chemical bonds, molecular orbitals (AO) are formed from atomic orbitals.
2. The number of obtained molecular orbitals is equal to the number of initial atomic ones.
3. Atomic orbitals that are close in energy overlap. As a result of the overlap of two atomic orbitals, two molecular orbitals are formed. One of them has a lower energy compared to the original atomic ones and is called binding , and the second molecular orbital has more energy than the original atomic orbitals, and is called loosening .
4. When atomic orbitals overlap, the formation of both -bonds (overlapping along the chemical bond axis) and -bonds (overlapping on both sides of the chemical bond axis) is possible.
5. A molecular orbital that is not involved in the formation of a chemical bond is called non-binding . Its energy is equal to the energy of the original AO.
6. On one molecular orbital (as well as atomic orbital) it is possible to find no more than two electrons.
7. Electrons occupy the molecular orbital with the lowest energy (principle of least energy).
8. The filling of degenerate (with the same energy) orbitals occurs sequentially with one electron for each of them.
Let us apply the MO LCAO method and analyze the structure of the hydrogen molecule. Let us depict the energy levels of the atomic orbitals of the initial hydrogen atoms on two parallel diagrams (Fig. 3.5).
It can be seen that there is a gain in energy compared to unbound atoms. Both electrons lowered their energy, which corresponds to the unit of valence in the method of valence bonds (a bond is formed by a pair of electrons).
The MO LCAO method makes it possible to visually explain the formation of ions and , which causes difficulties in the method of valence bonds. One electron of the H atom passes to the -bonding molecular orbital of the cation with a gain in energy (Fig. 3.7).
In an anion, three electrons must already be placed in two molecular orbitals (Fig. 3.8).
If two electrons, having descended to the bonding orbital, give a gain in energy, then the third electron has to increase its energy. However, the energy gained by two electrons is greater than that lost by one. Such a particle may exist.
It is known that alkali metals in the gaseous state exist in the form of diatomic molecules. Let us try to verify the possibility of the existence of a diatomic Li 2 molecule using the MO LCAO method. The original lithium atom contains electrons at two energy levels - the first and second (1 s and 2 s) (Fig. 3.9).
Overlapping identical 1 s-orbitals of lithium atoms will give two molecular orbitals (bonding and loosening), which, according to the principle of minimum energy, will be completely populated by four electrons. The gain in energy resulting from the transition of two electrons to the bonding molecular orbital is not able to compensate for its losses during the transition of two other electrons to the antibonding molecular orbital. That is why only the electrons of the outer (valence) electron layer contribute to the formation of a chemical bond between lithium atoms.
Overlapping valence 2 s-orbitals of lithium atoms will also lead to the formation of one
-bonding and one loosening molecular orbitals. The two outer electrons will occupy the bonding orbital, providing an overall gain in energy (the bond multiplicity is 1).
Using the MO LCAO method, consider the possibility of the formation of the He 2 molecule (Fig. 3.10).
In this case, two electrons will occupy the bonding molecular orbital, and the other two will occupy the loosening orbital. Such a population of two orbitals with electrons will not bring a gain in energy. Therefore, the He 2 molecule does not exist.
Using the MO LCAO method, it is easy to demonstrate the paramagnetic properties of the oxygen molecule. In order not to clutter up the figure, we will not consider overlap 1 s-orbitals of oxygen atoms of the first (inner) electron layer. We take into account that p-orbitals of the second (outer) electron layer can overlap in two ways. One of them will overlap with a similar one with the formation of a -bond (Fig. 3.11).
Two others p-AO overlap on both sides of the axis x with the formation of two -bonds (Fig. 3.12).
The energies of the constructed molecular orbitals can be determined from the data of the absorption spectra of substances in the ultraviolet region. So, among the molecular orbitals of the oxygen molecule formed as a result of overlapping p-AO, two -bonding degenerate (with the same energy) orbitals have less energy than the -bonding one, however, like the *-loosening orbitals, they have less energy in comparison with the *-loosening orbital (Fig. 3.13).
In the O 2 molecule, two electrons with parallel spins ended up in two degenerate (with the same energy) *-loosening molecular orbitals. It is the presence of unpaired electrons that determines the paramagnetic properties of the oxygen molecule, which will become noticeable if oxygen is cooled to a liquid state.
Among the diatomic molecules, one of the strongest is the CO molecule. The MO LCAO method makes it easy to explain this fact (Fig. 3.14, see p. 18).
The result of the overlap p-orbitals of the O and C atoms is the formation of two degenerate
-bonding and one -bonding orbital. These molecular orbitals will occupy six electrons. Therefore, the multiplicity of the bond is three.
The MO LCAO method can be used not only for diatomic molecules, but also for polyatomic ones. Let us analyze, as an example, within the framework of this method, the structure of the ammonia molecule (Fig. 3.15).
Since three hydrogen atoms have only three 1 s-orbitals, then the total number of formed molecular orbitals will be equal to six (three bonding and three loosening). Two electrons of the nitrogen atom will be in a non-bonding molecular orbital (lone electron pair).
3.5. Geometric shapes of molecules
When talking about the shapes of molecules, first of all, they mean the relative position in space of the nuclei of atoms. It makes sense to talk about the shape of a molecule when the molecule consists of three or more atoms (two nuclei are always on the same straight line). The shape of molecules is determined on the basis of the theory of repulsion of valence (external) electron pairs. According to this theory, the molecule will always take the form in which the repulsion of external electron pairs is minimal (the principle of minimum energy). In doing so, the following assertions of the theory of repulsion must be borne in mind.
1. Lone electron pairs undergo the greatest repulsion.
2. The repulsion between the unshared pair and the pair involved in bond formation is somewhat less.
3. Least repulsion between the electron pairs involved in bond formation. But even this is not enough to separate the nuclei of atoms involved in the formation of chemical bonds to the maximum angle.
As an example, consider the forms of hydrogen compounds of elements of the second period: BeH 2, BH 3, CH 4, C 2 H 4, C 2 H 2, NH 3, H 2 O.
Let's start by determining the shape of the BeH 2 molecule. Let's depict its electronic formula:
from which it is clear that there are no unshared electron pairs in the molecule. Therefore, for electron pairs that bind atoms, it is possible to repel to the maximum distance at which all three atoms are on the same straight line, i.e. the HBeH angle is 180°.
The BH 3 molecule consists of four atoms. According to its electronic formula, there are no lone pairs of electrons in it:
The molecule will acquire such a shape in which the distance between all bonds is maximum, and the angle between them is 120°. All four atoms will be in the same plane - the molecule is flat:
The electronic formula of the methane molecule is as follows:
All atoms of a given molecule cannot be in the same plane. In this case, the angle between the bonds would be 90°. There is a more optimal (from an energy point of view) arrangement of atoms - tetrahedral. The angle between the bonds in this case is 109°28".
The electronic formula of ethene is:
Naturally, all angles between chemical bonds take on a maximum value of 120°.
Obviously, in an acetylene molecule, all atoms must be on the same straight line:
H:C:::C:H.
The difference between the ammonia molecule NH 3 and all the previous ones is the presence in it of a lone pair of electrons at the nitrogen atom:
As already mentioned, the electron pairs involved in bond formation are more strongly repelled from the lone electron pair. The lone pair is located symmetrically with respect to the hydrogen atoms in the ammonia molecule:
The HNH angle is smaller than the HCH angle in the methane molecule (due to the stronger electron repulsion).
There are already two lone pairs in a water molecule:
This is due to the angular shape of the molecule:
As a consequence of the stronger repulsion of lone electron pairs, the HOH angle is even smaller than the HNH angle in the ammonia molecule.
The given examples quite clearly demonstrate the possibilities of the theory of repulsion of valence electron pairs. It makes it relatively easy to predict the shapes of many inorganic and organic molecules.
3.6. Exercises
1
. What types of bonds can be classified as chemical?
2.
What are the two main approaches to the consideration of chemical bonds do you know? What is their difference?
3.
Define valency and oxidation state.
4.
What are the differences between simple covalent, donor-acceptor, dative, metallic, ionic bonds?
5.
How are intermolecular bonds classified?
6.
What is electronegativity? From what data is electronegativity calculated? What do the electronegativity of atoms forming a chemical bond allow us to judge? How does the electronegativity of atoms of elements change when moving in the periodic table of D.I. Mendeleev from top to bottom and from left to right?
7.
What rules should be followed when considering the structure of molecules by the MO LCAO method?
8.
Using the method of valence bonds, explain the structure of hydrogen compounds of elements
2nd period.
9.
The dissociation energy in the series of Cl 2, Br 2, I 2 molecules decreases (239 kJ/mol, 192 kJ/mol, 149 kJ/mol, respectively), but the dissociation energy of the F 2 molecule (151 kJ/mol) is much less than the dissociation energy Cl 2 molecules, and falls out of the general pattern. Explain the given facts.
10.
Why, under normal conditions, CO 2 is a gas, and SiO 2 is a solid, H 2 O is a liquid,
and H 2 S is a gas? Try to explain the state of aggregation of substances.
11.
Using the MO LCAO method, explain the occurrence and features of the chemical bond in the molecules B 2 , C 2 , N 2 , F 2 , LiH, CH 4 .
12.
Using the theory of repulsion of valence electron pairs, determine the shapes of the molecules of oxygen compounds of elements of the 2nd period.