Ionic bond. How an ionic bond is formed: examples Ionic type of chemical bond examples
Ionic bond
Chemical bond theory takes important place in modern chemistry. She explains why atoms combine to form chemical particles, And makes it possible to compare the stability of these particles. Using chemical bond theory, Can predict the composition and structure of various compounds. The concept of the breaking of some chemical bonds and the formation of others underlies modern ideas about the transformations of substances in the course of chemical reactions .
chemical bond- This interaction of atoms , determining the stability of a chemical particle or crystal as a whole . chemical bond formed through electrostatic interaction between charged particles : cations and anions, nuclei and electrons. When atoms approach each other, attractive forces begin to act between the nucleus of one atom and the electrons of another, as well as repulsive forces between nuclei and between electrons. On some distance these forces balance each other, And a stable chemical particle is formed .
When a chemical bond is formed, a significant redistribution of the electron density of atoms in the compound can occur compared to free atoms.
In the limiting case, this leads to the formation of charged particles - ions (from the Greek "ion" - going).
1 Interaction of ions
If atom loses one or few electrons, then he becomes a positive ion - cation(translated from Greek - " going down"). This is how cations hydrogen H +, lithium Li +, barium Ba 2+ . Acquiring electrons, atoms turn into negative ions - anions(from the Greek "anion" - going up). Examples of anions are fluoride ion F − , sulfide ion S 2− .
Cations And anions able attract each other. This gives rise to chemical bond, And chemical compounds are formed. This type of chemical bond is called ionic bond :
2 Ionic bond definition
Ionic bond is a chemical bond educated at the expense electrostatic attraction between cations And anions .
The mechanism of formation of an ionic bond can be considered on the example of the reaction between sodium and chlorine . An alkali metal atom easily loses an electron, A halogen atom - acquires. As a result of this, there sodium cation And chloride ion. They form a connection through electrostatic attraction between them .
Interaction between cations And anions does not depend on direction, That's why about ionic bond they talk about non-directional. Every cation Maybe attract any number of anions, And vice versa. That's why ionic bond is unsaturated. Number interactions between ions in the solid state is limited only by the size of the crystal. That's why " molecule " ionic compound should be considered the whole crystal .
For the emergence ionic bond necessary, to sum of ionization energies E i(to form a cation) And electron affinity Ae(for anion formation) must be energetically profitable. This limits the formation of ionic bonds by atoms of active metals(elements of IA- and IIA-groups, some elements of the IIIA-group and some transitional elements) and active non-metals(halogens, chalcogens, nitrogen).
Ideal ionic bond practically does not exist. Even in those compounds that are usually referred to as ionic , there is no complete transfer of electrons from one atom to another ; electrons partially remain in common use. Yes, the connection lithium fluoride by 80% ionic, and by 20% - covalent. Therefore, it is more correct to speak of degree of ionicity (polarity) covalent chemical bond. It is believed that with a difference electronegativity elements 2.1 communication is on 50% ionic. At greater difference compound can be considered ionic .
The ionic model of a chemical bond is widely used to describe the properties of many substances., in the first place, connections alkaline And alkaline earth metals with non-metals. This is due ease of description of such compounds: believe they are built from incompressible charged spheres, corresponding cations and anions. In this case, the ions tend to arrange themselves in such a way that the attractive forces between them are maximum, and the repulsive forces are minimal.
Ionic bond- a strong chemical bond formed between atoms with a large difference (>1.7 on the Pauling scale) of electronegativity, with which the shared electron pair goes entirely to the atom with the greater electronegativity. This is the attraction of ions as oppositely charged bodies. An example is the compound CsF, in which the "degree of ionicity" is 97%.
Ionic bond- extreme case polarization of a covalent polar bond. Formed between typical metal and non-metal. In this case, the electrons in the metal completely go to non-metal . Ions are formed.
If a chemical bond is formed between atoms that have very large electronegativity difference (EO > 1.7 according to Pauling), then the shared electron pair is completely goes to an atom with a higher EC. This results in the formation of a compound oppositely charged ions :
Between the formed ions there is electrostatic attraction, which is called ionic bond. Rather, this view convenient. In practice ionic bond between the atoms in in its pure form is not realized anywhere or almost nowhere, usually in reality the connection is partially ionic , and partially covalent character. At the same time, communication complex molecular ions can often be considered purely ionic. The most important differences between ionic bonds and other types of chemical bonds are non-directionality and unsaturation. That is why crystals formed due to ionic bonding gravitate towards various close packings of the corresponding ions.
3 Ionic radii
In idle electrostatic model of ionic bond concept is used ionic radii . The sum of the radii of the neighboring cation and anion must be equal to the corresponding internuclear distance :
r 0 = r + + r −
At the same time, it remains obscure where to take boundary between cation and anion . Known today , that a purely ionic bond does not exist, as always there is some electron cloud overlap. For ion radii calculations use research methods, which allow you to determine the electron density between two atoms . The internuclear distance is divided at a point, Where electron density is minimal .

Ion size depends on many factors. At constant charge of the ion with increasing serial number(and consequently, nuclear charge) ionic radius decreases. This is especially noticeable in the lanthanide series, Where ionic radii change monotonically from 117 pm for (La 3+) to 100 pm (Lu 3+) at a coordination number of 6. This effect is called lanthanide compression .
IN element groups ionic radii generally increase with increasing atomic number. However For d-elements of the fourth and fifth periods due to lanthanide compression even a decrease in the ionic radius can occur(for example, from 73 pm for Zr 4+ to 72 pm for Hf 4+ with a coordination number of 4).
In the period, there is a noticeable decrease in the ionic radius associated with an increase in the attraction of electrons to the nucleus with a simultaneous increase in the charge of the nucleus and the charge of the ion itself: 116 pm for Na +, 86 pm for Mg 2+ , 68 pm for Al 3+ (coordination number 6). For the same reason an increase in the charge of an ion leads to a decrease in the ionic radius for one element: Fe 2+ 77 pm, Fe 3+ 63 pm, Fe 6+ 39 pm (coordination number 4).
Comparison ionic radii Can carried out only with the same coordination number, because the it affects the size of the ion due to the repulsive forces between the counterions. This is clearly seen in the example Ag+ ion; its ionic radius is 81, 114 and 129 pm For coordination numbers 2, 4 and 6 , respectively .
Structure perfect ionic compound, due to maximum attraction between dissimilar ions and minimum repulsion between like ions, in many ways determined by the ratio of the ionic radii of cations and anions. It can be shown simple geometric constructions.
4 Ionic bond energy
Bond energy And for ionic compound- This energy, which in is released during its formation from gaseous counterions infinitely distant from each other . Considering only electrostatic forces corresponds to about 90% of the total interaction energy, which includes also the contribution of non-electrostatic forces(For example, repulsion of electron shells).
When ionic bond between two free ion energy their attraction is determined by Coulomb's law :
E(adj.) = q+ q− / (4π r ε),
Where q+ And q−- charges interacting ions , r - the distance between them , ε - medium permittivity .
Since one of the charges negative, That energy value Also will be negative .
According to Coulomb's law, on At infinitesimal distances, the energy of attraction must become infinitely large. However, this not happening, because ions are not point charges. At approach of ions there is a repulsive force between them, due to interaction of electron clouds . Ion repulsion energy described Born equation :
E (ott.) \u003d B / rn,
Where IN - some constant , n Maybe take values from 5 to 12(depends on ion size). The total energy is determined by the sum of the energies of attraction and repulsion :
E \u003d E (adv.) + E (ott.)
Its meaning goes through minimum . The coordinates of the minimum point correspond to the equilibrium distance r 0 And equilibrium energy of interaction between ions E 0 :
E0 = q+ q− (1 - 1 / n) / (4π r0 ε)
IN crystal lattice Always there are more interactions, how between a pair of ions. This number determined primarily by the type of crystal lattice. For accounting for all interactions(weakening with increasing distance) into the expression for ionic energy crystal lattice introduce the so-called constant Madelunga A :
E(adj.) = A q+ q− / (4π r ε)
Constant value Madelunga determined only lattice geometry and not depends on the radius and charge of the ions. For example, for sodium chloride it is equal to 1,74756 .
5 polarization of ions
Apart from charge magnitude And radius important characteristic and she are his polarization properties. Let's consider this question in more detail. At non-polar particles (atoms, ions, molecules) the centers of gravity of positive and negative charges coincide. In an electric field, the electron shells are displaced in the direction of a positively charged plate, and nuclei - in the direction of a negatively charged plate. Due to particle deformation arises in it dipole, she becomes polar .
source electric field in compounds with an ionic type of bond are the ions themselves. Therefore, speaking of polarization properties of the ion , necessary distinguish the polarizing effect of a given ion And the ability of itself to polarize in an electric field .
The polarizing effect of the ion will be the one big, how more of his force field, i.e. than more charge and less ion radius. Therefore, in within subgroups in the Periodic Table of the Elements the polarizing effect of ions decreases from top to bottom, because in subgroups with a constant value of the charge of the ion from top to bottom, its radius increases .
That's why the polarizing effect of alkali metal ions, for example, increases from cesium to lithium, and in a row halide ions - from I to F. In periods the polarizing effect of the ions increases from left to right together with an increase in the charge of the ion And decreasing its radius .
Ion polarizability, its ability to deformations increase with decreasing force field, i.e. with a decrease in the amount of charge And increase in radius . Anion polarizability usually higher, how cations and in a row halides grows from F to I .
On polarization properties of cations renders influence the nature of their outer electron shell . Polarization properties of cations how in active, as well as in passive sense at the same charge and a close radius increase upon passing from cations with a filled shell to cations with an incomplete outer shell and further to cations with an 18-electron shell.
For example, in the series of cations Mg 2+ , Ni 2+ , Zn 2+ polarization properties intensify. This pattern is consistent with the change in the ion radius and the structure of its electron shell given in the series:
for anions polarization properties deteriorate in this order:
I - , Br - , Cl - , CN - , OH - , NO 3 - , F - , ClO 4 - .
result polarization interaction of ions is deformation of their electron shells and, as a consequence of this, shortening of interionic distances And incomplete separation of the negative And positive charges between ions.
For example, in a crystal sodium chloride the amount of charge on sodium ion is +0,9 , and on chlorine ion - 0.9 instead of expected unit. In a molecule KCl located in vapor state, value charges on potassium ions And chlorine is 0.83 charge units, and in the molecule hydrogen chloride- only 0,17 units of charge.
Ion polarization renders noticeable effect on the properties of compounds with ionic bond , lowering their melting and boiling points , reducing electrolytic dissociation in solutions and melts, etc. .
Ionic compounds formed when interaction of elements , significantly different in chemical properties. The more the distance between elements in the periodic table, topics in ionic bond is more pronounced in their compounds . Against, in molecules, formed by the same atoms or atoms of elements that are similar in chemical properties, arise other types of communication. That's why ionic bond theory It has limited use .
6 Effect of ion polarization on the properties of substances and properties of ionic bonds and ionic compounds
Ideas about ion polarizations help explain differences in the properties of many similar substances. For example, comparison sodium chloride And potassium with silver chloride shows that when close ionic radii
polarizability of the Ag+ cation having 18-electron outer shell , higher, What leads to an increase in the metal-chlorine bond strength And lower solubility of silver chloride in water .
Mutual polarization of ions facilitates the destruction of crystals, that leads to lowering the melting points of substances. For this reason melting temperature TlF (327 oС) significantly lower than RbF (798 oC). The decomposition temperature of substances will also decrease with an increase in the mutual polarization of ions. That's why iodides generally decompose at lower temperatures, how other halides, A lithium compounds - thermally less stable , than compounds of other alkaline elements .
Deformability of electron shells affects the optical properties of substances. How more polarized particle , the lower the energy of electronic transitions. If polarization is low , excitation of electrons requires higher energy, which answers ultraviolet part of the spectrum. Such substances are usually colorless. In the case of strong polarization of ions, the excitation of electrons occurs upon absorption of electromagnetic radiation in the visible region of the spectrum. That's why some substances, formed colorless ions, colored .
characteristic ionic compounds serves good solubility in polar solvents (water, acids, etc.). This is due to the charge of the parts of the molecule. Wherein solvent dipoles are attracted to the charged ends of the molecule, and as a result brownian motion , « take away» molecule substances into parts and surround them , preventing reconnection. The result is ions surrounded by solvent dipoles .
When such compounds are dissolved, as a rule, energy is released, since the total energy of the formed bonds solvent-ion has more anion-cation bond energy. Exceptions are many salts of nitric acid (nitrates), which absorb heat when dissolved (solutions are cooled). The latter fact is explained on the basis of the laws that considered in physical chemistry .
7 Crystal lattice
Ionic compounds(e.g. sodium chloride NaCl) - solid And refractory because of between the charges of their ions("+" and "-") exist powerful forces of electrostatic attraction .
The negatively charged chloride ion attracts Not only " mine " Na+ ion, but also other sodium ions around. This leads to, What near any of the ions there is more than one ion with the opposite sign , but a few(Fig. 1).

Rice. 1. Crystal structure common salt NaCl .
In fact, about every chloride ion is located 6 sodium ions, and about each sodium ion - 6 chloride ions .
This ordered packing of ions is called ionic crystal. If we single out a separate chlorine atom, then among surrounding sodium atoms already impossible to find one, which chlorine reacted.. Drawn to each other electrostatic forces , ions are extremely reluctant to change their location under the influence of an external force or temperature increase. But if the temperature is very high (approx. 1500°C), That NaCl evaporates, forming diatomic molecules. This suggests that covalent bonding forces never turn off completely .
Ionic crystals different high melting points, usually significant band gap, possess ionic conductivity at high temperatures And a number of specific optical properties(For example, transparency in the near IR spectrum). They can be built from monatomic, and from polyatomic ions. Example ionic crystals of the first type - alkali halide crystals And alkaline earth metals ; anions are arranged according to the law of closest spherical packing or dense ball masonry , cations occupy the corresponding voids. Most characteristic structures of this type are NaCl, CsCl, CaF2. Ionic crystals of the second type built from monatomic cations of the same metals and finite or infinite anionic fragments . Terminal anions(acid residues) - NO3-, SO42-, CO32- and others . Acidic residues can form endless chains , layers or form a three-dimensional frame, in the cavities of which cations are located, as, for example, in crystal structures of silicates. For ionic crystals it is possible to calculate the energy of the crystal structure U(see table), approximately equal to enthalpy of sublimation; results are in good agreement with the experimental data. According to the equation Born-Meyer, For crystal, consisting of formally singly charged ions :
U \u003d -A / R + Be-R / r - C / R6 - D / R8 + E0
(R - shortest inter-ion distance , A - Madelung constant , dependent from structure geometry , IN And r - options , describing repulsion between particles , C/R6 And D/R8 characterize the respective dipole-dipole and dipole-quadrupole interaction of ions , E 0 - zero point energy , e - electron charge). WITH as the cation grows larger, the contribution of dipole-dipole interactions increases .
All chemical compounds are formed through the formation of a chemical bond. And depending on the type of connecting particles, several types are distinguished. The most basic- these are covalent polar, covalent non-polar, metallic and ionic. Today we will talk about ionic.
In contact with
What are ions
It is formed between two atoms - as a rule, provided that the difference in electronegativity between them is very large. Electronegativity of atoms and ions is estimated according to the Polling scale.
Therefore, in order to correctly consider the characteristics of compounds, the concept of ionicity was introduced. This characteristic allows you to determine how many percent a particular bond is ionic.
The compound with the highest ionicity is cesium fluoride, in which it is approximately 97%. Ionic bond is characteristic for substances formed by metal atoms located in the first and second groups of the table D.I. Mendeleev, and atoms of non-metals in the sixth and seventh groups of the same table.
Note! It is worth noting that there is no compound in which the relationship is exclusively ionic. For currently discovered elements, it is impossible to achieve such a large difference in electronegativity as to obtain a 100% ionic compound. Therefore, the definition of an ionic bond is not entirely correct, since compounds with partial ionic interaction are actually considered.
Why was this term introduced, if such a phenomenon does not really exist? The fact is that this approach helped to explain many nuances in the properties of salts, oxides and other substances. For example, why are they highly soluble in water, and their solutions are capable of conducting electricity. It cannot be explained from any other position.
Mechanism of education
The formation of an ionic bond is possible only if two conditions are met: if the metal atom participating in the reaction is able to easily donate electrons that are at the last energy level, and the non-metal atom is able to accept these electrons. Metal atoms are inherently reducing agents, that is, they are capable of recoil of electrons.
This is due to the fact that at the last energy level in the metal there can be from one to three electrons, and the radius of the particle itself is quite large. Therefore, the force of interaction of the nucleus with electrons at the last level is so small that they can easily leave it. With non-metals, the situation is completely different. They have small radius, and the number of own electrons at the last level can be from three to seven.
 And the interaction between them and the positive nucleus is quite strong, but any atom tends to complete the energy level, so non-metal atoms tend to get the missing electrons.
And the interaction between them and the positive nucleus is quite strong, but any atom tends to complete the energy level, so non-metal atoms tend to get the missing electrons.
And when two atoms meet - a metal and a non-metal, there is a transition of electrons from the metal atom to the non-metal atom, and a chemical interaction is formed.
Connection diagram
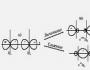
The figure clearly shows how the formation of an ionic bond is carried out. Initially, there are neutrally charged atoms of sodium and chlorine.
The first has one electron in the last energy level, the second has seven. Next, an electron passes from sodium to chlorine and the formation of two ions. Which combine with each other to form a substance. What is an ion? An ion is a charged particle that the number of protons is not equal to the number of electrons.
Differences from the covalent type
 The ionic bond, due to its specificity, has no direction. This is due to the fact that the electric field of an ion is a sphere, while it decreases or increases uniformly in one direction, obeying the same law.
The ionic bond, due to its specificity, has no direction. This is due to the fact that the electric field of an ion is a sphere, while it decreases or increases uniformly in one direction, obeying the same law.
Unlike covalent, which is formed due to the overlap of electron clouds.
The second difference is that covalent bond is saturated. What does it mean? The number of electronic clouds that can take part in the interaction is limited.
And in the ion one, due to the fact that the electric field has a spherical shape, it can combine with an unlimited number of ions. So, we can say that it is not saturated.
It can also be characterized by several other properties:
- The bond energy is a quantitative characteristic, and it depends on the amount of energy that must be expended to break it. It depends on two criteria - bond length and ion charge involved in its formation. The bond is stronger, the shorter its length and the greater the charges of the ions that form it.
- Length - this criterion has already been mentioned in the previous paragraph. It depends solely on the radius of the particles involved in the formation of the compound. The radius of atoms changes as follows: decreases in period with increasing serial number and increases in the group.
Substances with an ionic bond
It is characteristic of a significant number of chemical compounds. This is a large part of all salts, including the well-known table salt. It occurs in all compounds where there is a direct contact between metal and non-metal. Here are some examples of substances with an ionic bond:
- sodium and potassium chlorides,
- cesium fluoride,
- magnesium oxide.
 It can also appear in complex compounds.
It can also appear in complex compounds.
For example, magnesium sulfate.
Here is the formula of a substance with ionic and covalent bonds:
An ionic bond will form between oxygen and magnesium ions, but sulfur and are interconnected already with the help of a covalent polar one.
From which we can conclude that the ionic bond is characteristic of complex chemical compounds.
What is an ionic bond in chemistry
Types of chemical bond - ionic, covalent, metallic
Conclusion
Properties directly dependent on the device crystal lattice. Therefore, all compounds with an ionic bond are highly soluble in water and other polar solvents, conduct and are dielectrics. At the same time, they are quite refractory and brittle. The properties of these substances are often used in the construction of electrical appliances.
Ions are atoms that have lost or gained electrons and, as a result, some charge. To begin with, I would like to recall that ions are of two types: cations(the positive charge of the nucleus is greater than the number of electrons carrying the negative charge) and anions(the charge of the nucleus is less than the number of electrons). An ionic bond is formed as a result of the interaction of two ions with opposite charges.
Ionic and covalent bond
This type of bond is a special case of a covalent bond. The difference in electronegativity in this case is so large (more than 1.7 according to Pauling) that the common pair of electrons is not partially displaced, but completely transferred to an atom with a greater electronegativity. Therefore, the formation of an ionic bond is the result of a strong electrostatic interaction between ions. It is important to understand that there is no 100% ionic bond. This term is used if the "ionic features" are more pronounced (i.e., the electron pair is strongly shifted to a more electronegative atom).
Ionic bond mechanism
Atoms that have an almost complete or almost empty valence (outer) shell most readily enter into chemical reactions. The fewer empty orbitals in the valence shell, the higher the chance that the atom will receive electrons from outside. And vice versa - the fewer electrons are on the outer shell, the more likely it is that the atom will give up an electron.
ElectronegativityThis is the ability of an atom to attract electrons to itself, so atoms with the most filled valence shells are more electronegative.
A typical metal willingly gives up electrons, while a typical non-metal is more willing to take them. Therefore, most often ionic bonds are formed by metals and non-metals. Separately, another type of ionic bond should be mentioned - molecular. Its peculiarity is that not individual atoms, but whole molecules act as ions.
Ionic bond scheme
The figure schematically shows the formation of sodium fluoride. Sodium has a low electronegativity and only one electron per valence shell (VO). Fluorine has a much higher electronegativity, it lacks only one electron to fill the VO. An electron from the sodium VO passes to the fluorine VO, filling the orbital, as a result of which both atoms acquire opposite charges and are attracted to each other.
Ionic bond properties
The ionic bond is strong enough - it is extremely difficult to destroy it with the help of thermal energy, and therefore substances with an ionic bond have high melting point. At the same time, the ion interaction radius is quite low, which causes brittleness similar connections. Its most important properties are non-directionality and unsaturation. The non-directionality comes from the shape of the ion's electric field, which is a sphere and is able to interact with cations or anions in all directions. In this case, the fields of two ions are not fully compensated, as a result of which they are forced to attract additional ions to themselves, forming a crystal - this is the phenomenon called unsaturation. There are no molecules in ionic crystals, and individual cations and anions are surrounded by many ions of the opposite sign, the number of which depends mainly on the position of the atoms in space.
Salt crystals (NaCl) are a typical example of an ionic bond.
Ionic bond
(materials of the website http://www.hemi.nsu.ru/ucheb138.htm were used)
Ionic bonding is carried out by electrostatic attraction between oppositely charged ions. These ions are formed as a result of the transfer of electrons from one atom to another. An ionic bond is formed between atoms that have large differences in electronegativity (usually greater than 1.7 on the Pauling scale), for example, between alkali metals and halogens.
Let us consider the appearance of an ionic bond using the example of the formation of NaCl.
From the electronic formulas of atoms
Na 1s 2 2s 2 2p 6 3s 1 and
Cl 1s 2 2s 2 2p 6 3s 2 3p 5
It can be seen that to complete the external level, it is easier for the sodium atom to give up one electron than to add seven, and for the chlorine atom it is easier to add one than to give up seven. In chemical reactions, the sodium atom donates one electron, and the chlorine atom accepts it. As a result, the electron shells of sodium and chlorine atoms are converted into stable electron shells of noble gases (the electronic configuration of the sodium cation
Na + 1s 2 2s 2 2p 6 ,
and the electronic configuration of the chlorine anion
Cl – - 1s 2 2s 2 2p 6 3s 2 3p 6).
The electrostatic interaction of ions leads to the formation of the NaCl molecule.
The nature of the chemical bond is often reflected in the state of aggregation and the physical properties of the substance. Ionic compounds such as sodium chloride NaCl are solid and refractory because there are powerful forces of electrostatic attraction between the charges of their "+" and "-" ions.
A negatively charged chloride ion attracts not only "its own" Na + ion, but also other sodium ions around it. This leads to the fact that near any of the ions there is not one ion with the opposite sign, but several.
The structure of the sodium chloride NaCl crystal.
In fact, there are 6 sodium ions around each chloride ion, and 6 chloride ions around each sodium ion. Such an ordered packing of ions is called an ionic crystal. If a separate chlorine atom is isolated in a crystal, then among the surrounding sodium atoms it is no longer possible to find the one with which chlorine reacted.
Attracted to each other by electrostatic forces, the ions are extremely reluctant to change their location under the influence of an external force or an increase in temperature. But if sodium chloride is melted and continued to be heated in a vacuum, then it evaporates, forming diatomic NaCl molecules. This suggests that covalent bonding forces are never completely turned off.
Main characteristics of ionic bond and properties of ionic compounds
1. An ionic bond is a strong chemical bond. The energy of this bond is about 300 – 700 kJ/mol.
2. Unlike a covalent bond, an ionic bond is non-directional, since an ion can attract ions of the opposite sign to itself in any direction.
3. Unlike a covalent bond, an ionic bond is unsaturated, since the interaction of ions of the opposite sign does not lead to complete mutual compensation of their force fields.
4. In the process of formation of molecules with an ionic bond, there is no complete transfer of electrons, therefore, a 100% ionic bond does not exist in nature. In the NaCl molecule, the chemical bond is only 80% ionic.
5. Ionic compounds are crystalline solids with high melting and boiling points.
6. Most ionic compounds dissolve in water. Solutions and melts of ionic compounds conduct electric current.
metal connection
Metal crystals are arranged differently. If you consider a piece of metallic sodium, you will find that outwardly it is very different from table salt. Sodium is a soft metal, easily cut with a knife, flattened with a hammer, it can be easily melted in a cup on a spirit lamp (melting point 97.8 o C). In a sodium crystal, each atom is surrounded by eight other similar atoms.

The structure of the crystal of metallic Na.
It can be seen from the figure that the Na atom in the center of the cube has 8 nearest neighbors. But the same can be said about any other atom in a crystal, since they are all the same. The crystal consists of "infinitely" repeating fragments shown in this picture.
Metal atoms at the outer energy level contain a small number of valence electrons. Since the ionization energy of metal atoms is low, valence electrons are weakly retained in these atoms. As a result, positively charged ions and free electrons appear in the crystal lattice of metals. In this case, the metal cations are located in the nodes of the crystal lattice, and the electrons move freely in the field of positive centers, forming the so-called "electron gas".
The presence of a negatively charged electron between two cations leads to the fact that each cation interacts with this electron.
Thus, a metallic bond is a bond between positive ions in metal crystals, which is carried out by the attraction of electrons that move freely throughout the crystal.
Since the valence electrons in the metal are evenly distributed throughout the crystal, the metallic bond, like the ionic one, is an undirected bond. Unlike a covalent bond, a metallic bond is an unsaturated bond. A metallic bond differs from a covalent bond in strength as well. The energy of a metallic bond is about three to four times less than the energy of a covalent bond.
Due to the high mobility of the electron gas, metals are characterized by high electrical and thermal conductivity.
A metal crystal looks simple enough, but its electronic structure is actually more complex than that of ionic salt crystals. There are not enough electrons on the outer electron shell of metal elements to form a full-fledged "octet" covalent or ionic bond. Therefore, in the gaseous state, most metals consist of monatomic molecules (i.e., individual, unrelated atoms). A typical example is mercury vapor. Thus, a metallic bond between metal atoms occurs only in the liquid and solid state of aggregation.
A metallic bond can be described as follows: some of the metal atoms in the resulting crystal give up their valence electrons to the space between the atoms (in sodium it is ... 3s1), turning into ions. Since all metal atoms in a crystal are the same, each of them has an equal chance of losing a valence electron.
In other words, the transition of electrons between neutral and ionized metal atoms occurs without energy consumption. In this case, a part of the electrons always ends up in the space between the atoms in the form of an "electron gas".
These free electrons, firstly, hold the metal atoms at a certain equilibrium distance from each other.
Secondly, they give metals a characteristic "metallic sheen" (free electrons can interact with light quanta).
Thirdly, free electrons provide metals with good electrical conductivity. The high thermal conductivity of metals is also explained by the presence of free electrons in the interatomic space - they easily "respond" to changes in energy and contribute to its rapid transfer in the crystal.

A simplified model of the electronic structure of a metal crystal.
******** On the example of sodium metal, let us consider the nature of the metallic bond from the point of view of ideas about atomic orbitals. The sodium atom, like many other metals, has a lack of valence electrons, but there are free valence orbitals. The only 3s electron of sodium is able to move to any of the free and close in energy neighboring orbitals. When atoms in a crystal approach each other, the outer orbitals of neighboring atoms overlap, due to which the donated electrons move freely throughout the crystal.
However, the "electron gas" is not at all disordered, as it might seem. Free electrons in a metal crystal are in overlapping orbitals and are socialized to some extent, forming a kind of covalent bonds. Sodium, potassium, rubidium, and other metallic s-elements simply have few shared electrons, so their crystals are fragile and fusible. With an increase in the number of valence electrons, the strength of metals, as a rule, increases.
Thus, elements tend to form a metallic bond, the atoms of which on the outer shells have few valence electrons. These valence electrons, which carry out the metallic bond, are so socialized that they can move throughout the entire metal crystal and provide a high electrical conductivity of the metal.
The NaCl crystal does not conduct electricity because there are no free electrons in the space between the ions. All electrons donated by sodium atoms firmly hold chloride ions around them. This is one of the essential differences between ionic crystals and metallic ones.
What you now know about the metallic bond also explains the high malleability (ductility) of most metals. Metal can be flattened into a thin sheet, pulled into a wire. The fact is that separate layers of atoms in a metal crystal can relatively easily slide one over another: the mobile "electron gas" constantly softens the movement of individual positive ions, shielding them from each other.
Of course, nothing of the kind can be done with table salt, although salt is also a crystalline substance. In ionic crystals, valence electrons are firmly bound to the nucleus of an atom. The shift of one layer of ions relative to another leads to the convergence of ions of the same charge and causes a strong repulsion between them, resulting in the destruction of the crystal (NaCl is a brittle substance).

The shift of the layers of the ionic crystal causes the appearance of large repulsive forces between like ions and the destruction of the crystal.
Navigation
- Solving combined problems based on the quantitative characteristics of a substance
- Problem solving. The law of the constancy of the composition of substances. Calculations using the concepts of "molar mass" and "chemical amount" of a substance
| Structural chemistry | |
|---|---|
| Chemical bond: | Aromaticity | Covalent bond | Ionic bond| Metal connection | Hydrogen bond | Donor-acceptor bond | Tautomerism |
| Structure display: | Functional group | Structural formula | Chemical formula | ligand |
| Electronic properties: | Electronegativity | Electron affinity | Ionization energy | Dipole | Octet rule |
| Stereochemistry: | Asymmetric atom | Isomerism | Configuration | Chirality | Conformation |
Wikimedia Foundation. 2010 .
See what "Ionic chemical bond" is in other dictionaries:
The bond between atoms in a molecule or mol. connection, arising as a result of either the transfer of an electron from one atom to another, or the socialization of electrons by a pair (or group) of atoms. The forces leading to X. s. are Coulomb, but X. s. describe within... Physical Encyclopedia
CHEMICAL BOND- interaction of atoms, in which electrons belonging to two different atoms (groups) become common (socialized) for both atoms (groups), causing their combination into molecules and crystals. There are two main types of X. s .: ionic ... ... Great Polytechnic Encyclopedia
CHEMICAL BOND The mechanism by which atoms combine to form molecules. There are several types of such a bond, based either on the attraction of opposite charges, or on the formation of stable configurations through the exchange of electrons. ... ... Scientific and technical encyclopedic dictionary
chemical bond- CHEMICAL BOND, the interaction of atoms, causing their connection into molecules and crystals. The forces acting during the formation of a chemical bond are mainly electrical in nature. The formation of a chemical bond is accompanied by a rearrangement ... ... Illustrated Encyclopedic Dictionary
- ... Wikipedia
Mutual attraction of atoms, leading to the formation of molecules and crystals. It is customary to say that in a molecule or in a crystal between neighboring atoms there are ch. The valency of an atom (which is discussed in more detail below) indicates the number of bonds ... Great Soviet Encyclopedia
chemical bond- mutual attraction of atoms, leading to the formation of molecules and crystals. The valence of an atom shows the number of bonds formed by a given atom with neighboring ones. The term "chemical structure" was introduced by Academician A. M. Butlerov in ... ... Encyclopedic Dictionary of Metallurgy
The interaction of atoms, which determines their connection into molecules and crystals. This interaction leads to a decrease in the total energy of the resulting molecule or crystal compared to the energy of non-interacting atoms and is based on ... ... Big encyclopedic polytechnic dictionary
Covalent bond on the example of a methane molecule: a complete external energy level for hydrogen (H) 2 electrons, and for carbon (C) 8 electrons. Covalent bond bond formed by directed valence electron clouds. Neutral ... ... Wikipedia
Chemical bonding is the phenomenon of the interaction of atoms, due to the overlap of electron clouds, binding particles, which is accompanied by a decrease in the total energy of the system. The term "chemical structure" was first introduced by A. M. Butlerov in 1861 ... ... Wikipedia