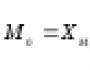Structural phenomenological model of composites with a thermoplastic matrix. Nonlinear Deformation of Two-Matrix Composite Structures
5.4.2. Polymer Matrix Composites
Composite materials with a polymer matrix are characterized by low density (1200 ... 1900 kg / m 3), low notch sensitivity, thermal and electrical conductivity, high fatigue and specific strength, processability, radio transparency (a number of materials), etc. As a polymer matrices for composites are used as thermosetting (mainly) and thermoplastic polymers, and fillers - any of the above.
Materials based on thermoplastic polymers with dispersed fillers of various nature (talc, graphite, metal oxides, layered solid lubricants, metal powders, discrete fiberglass, etc.) are used for the manufacture of lightly and medium-loaded machine and apparatus parts, body parts, gears and sprockets, bearings and seals, drive belts, containers, etc.
Among thermoplastic composites, glass-filled materials are the most widely used. As a filler, fibers with a diameter of 9 ... 13 microns from alkali-free aluminoborosilicate glass are used, short (0.1 ... 1 microns long) and long (3 ... 12 mm long) with a filling degree of 10 ... 40% of polymer mass. Glass-filled plastics based on polyamides, polycarbonate, polypropylene and other thermoplastics are produced. Filling thermoplastics with glass fiber increases the strength characteristics of polymers and heat resistance, reduces creep by 1.5 ... 2 times, reduces thermal expansion by 2 ... 7 times, increases endurance limit and wear resistance. The introduction of solid layered lubricants into composites, such as graphite, molybdenum disulfide, boron nitride, etc., reduces the coefficient of friction of polymers and increases their wear resistance.
The strength of composites based on thermoplastics reaches 150 ... 160 MPa with a sufficiently high impact strength (KCU = 8 ... 60 J/m2).
Composite materials based on thermosetting plastics are based on polymers that cure when heated or under the action of hardeners to form a three-dimensional polymer structure. Composites based on phenol-formaldehyde, urea-formaldehyde, melamine-formaldehyde, organosilicon and other resins are among those cured by heating. The second type includes composites based on polysiloxanes, epoxy resins, and unsaturated polyesters.
Thermosetting plastics, in contrast to thermoplastics, are characterized by a complete absence of cold flow, have significantly greater heat resistance, are insoluble, and have a slight swelling. They exhibit stability of properties up to the temperature of heat resistance, the ability to withstand long-term loads at temperatures from -60 to +200 ... 300 ° C, depending on the type of polymer, and have good dielectric properties. But these materials are less technologically advanced than thermoplastics.
Epoxy resins have the greatest adhesion to the filler. Cured epoxy resins are resistant to alkalis, oxidizing agents, and most organic acids. However, composites based on them have low mechanical properties, have heat resistance up to 200 °C, besides, these resins are toxic.
Composites based on organosilicon and polyimide binders have the highest heat resistance (up to 280 ... 350 °C).
The use of epoxy resins and unsaturated polyesters makes it possible to obtain materials capable of curing at room temperature (cold curing), which is very important in the manufacture of large-sized products.
Composite materials with dispersed fillers which are used as powders of organic (wood flour, cellulose) and mineral (quartz, talc, mica, metal oxides, solid layered lubricants, including graphite, molybdenum disulfide, boron nitride) substances, have isotropic properties, low mechanical strength and impact strength.
As fibrous reinforcing materials cotton tows, cord threads, asbestos fiber, fiberglass are used. Accordingly, these materials are called fibers, cord fibers, asbestos fibers, glass fibers.
fibers - plastics based on cotton linters impregnated with phenol-formaldehyde resin. The materials have an increased impact strength (up to 10 kJ/m 2 ) compared to press powders, but they have significantly lower fluidity, which does not allow obtaining thin-walled parts. Fibers have low dielectric properties, are unstable to tropical climates, and have anisotropic properties. They are used for the manufacture of general technical products with increased resistance to vibration and shock loads, working in bending and torsion, for example, belt pulleys, flanges, handles, covers, etc.
Asbestos fibers - composites containing a fibrous mineral - asbestos, splitting into thin fibers with a diameter of up to 0.5 microns. Phenol-formaldehyde and organosilicon resins are used as a binder. They have high impact strength and heat resistance up to 200 °C, are resistant to acidic environments, and have good frictional properties. They are mainly used as materials for brake devices (brake pads, linings, clutch discs).
Phenol-formaldehyde-based asbestos fibers are used for the production of high-strength heat-resistant parts for electrical purposes (electrical panels, high- and low-voltage collectors), and based on organosilicon polymers - for parts that operate for a long time at temperatures up to 200 ° C (material K-41-5) and for arcing chambers of high power contactors, terminal blocks (KMK-218). The latest materials are tropical resistant. Faolite - asb fiber, obtained by impregnating asb fibers with phenol-formaldehyde resin, followed by rolling the mixture, is used for the manufacture of acid-resistant pipes and containers.
Fiberglass are plastics containing fiberglass as a filler. Glass fibers with a diameter of 5 ... 20 microns are used, high-strength with a tensile strength V = 600 ... 3800 MPa and high-modulus (VM-1, VMP, M-11), having V = 3900 ... 4700 MPa and an elastic modulus at stretching up to 110 GPa. Fibers, threads, bundles of different lengths are used, which largely determines the impact strength of fiberglass. The thinner the fiber, the less its defects and the higher the strength.
The mechanical properties of glass fibers depend on the composition, quantity and length of glass fibers, the type of binder, physical and chemical processes occurring at the interface between fiberglass and binder, and the processing method. For example, replacing glass fiber from E glass (alkali-free aluminosilicate) with S glass fiber (heat-resistant high-strength) in an epoxy binder can increase the strength of the composite by 40%.
In order to improve the wettability of fiberglass with a binder, reduce the stresses that arise at the interface, increase adhesion between the fiber and the binder, dressing (treatment) of fibers with compounds containing various reactive groups (vinyl, methacrylic, phenyl, amino and imino groups, etc.) is used. Reduction of stresses in the boundary layer of the binder with the fiber, reduction of shrinkage and porosity, increase in heat resistance is facilitated by the introduction of powdered fillers into the binder, in particular, the powder of the cured binder.
Fiberglass is divided into: entangled fibrous, granular and finely dispersed press masses.
Tangled Fiberglass obtained by impregnation of fiber segments 40 ... 70 mm long, followed by fluffing and drying to remove the solvent (for example, AG-4V). The disadvantage of these materials is the uneven distribution of the binder, a greater spread of mechanical properties and less fluidity compared to other fiberglass.
Granular fiberglass(premixes) obtained by impregnation of untwisted glass fibers and glass bundles, followed by drying and cutting into granules 5, 10, 20 and 30 mm long. Granule diameter 0.5 ... 8 mm. The material has good flowability and fluidity, greater stability of mechanical properties. This category of materials includes dosing fiberglass DSV.
Fine glass fiber presses are made by mixing crushed glass fibers up to 1.5 mm long with a binder, followed by granulation (granules 3 ... 6 mm in size). Also produced "glass chips" with granules up to 10 ... 50 mm long from impregnated fiberglass waste.
Fiberglass granulated with granules up to 6 mm in size is processed by injection molding. Fine glass fibers can be processed by injection molding, and in the manufacture of products with metal fittings - injection molding. Fiberglass with a grain length of 10 mm is processed by casting and direct pressing, and with a grain length of 20 and 30 mm - only by direct pressing.
Fiberglass is used to make body parts, elements of shields, insulators, plug connectors, antenna fairings, etc. Products operated at temperatures from -60 to +200 °C are made on the basis of aniline-phenol-formaldehyde resins and alkali-free aluminoborosilicate glass fiber, and for the temperature range - 60 ... +100 °C on the basis of epoxy resins.
Glass fibers based on organosilicon resins are operated up to a temperature of 400 °C, and with the use of quartz or silica fibers for a short time and at higher temperatures. For heat-shielding parts, glass fibers based on silica fiber and phenol-formaldehyde resins are used.
Based on glass mats and unsaturated polyester resins, prepregs, which are used for the manufacture of large-sized parts (bodies, boats, body parts of devices, etc.). The use of oriented fibers makes it possible to obtain glass fibers with improved mechanical properties. For example, oriented fiberglass AG-4C has: V = 200 ... 400 MPa, KCU = 100 kJ / m 2; while for AG-4V based on tangled fiber: V = 80 MPa, KCU = 25 kJ / m 2.
Organic fibers are composite materials based on polymer binders, in which fibers of organic polymers (polyamide, lavsan, nitron, vinol, etc.) serve as a filler. Harnesses, fabrics and mats from these fibers are also used for reinforcement. Thermosetting resins (epoxy, phenol-formaldehyde, polyimide, etc.) are used as binders.
The use of polymer binders and fillers with similar thermophysical characteristics, as well as those capable of diffusion and chemical interaction between them, provides composites with the stability of mechanical properties, high specific strength and impact strength, chemical resistance, resistance to thermal shock, tropical atmosphere, and abrasion. The permissible operating temperature of most organo fibers is 100 ... 150 ° C, and on the basis of a polyimide binder and heat-resistant fibers - up to 200 ... 300 ° C. The disadvantages of these materials include low compressive strength and creep.
To obtain high-strength composites, fibers based on aromatic polyamides (aramid fibers SVM, Terlon, Kevlar) are used, which have high mechanical properties, thermal stability in a wide temperature range, good dielectric and fatigue properties. In terms of specific strength, these fibers are second only to boron and carbon fibers.
Boron fibers - composite materials on a polymer matrix filled with boron fibers. They have good mechanical properties, low creep, high thermal and electrical conductivity, resistance to organic solvents, fuels and lubricants, radioactive radiation, and cyclic alternating loads.
Boron fibers are produced by chemical deposition of boron from a BCl 3 +H 2 gas mixture onto a tungsten filament at a temperature of ~1130°C. To increase heat resistance, the fibers are coated with silicon carbide, which is also deposited from the vapor-gas phase in an argon and hydrogen environment. Such fibers are called borsik. As a binder for boron fibers, modified epoxy resins and polyimides are used. Boron fibers KMB-3, KMB-Zk ensure the performance of products at temperatures up to 100 °C, KMB-1 and KMB-1k up to 200 °C, and KMB-2k up to 300 °C. In order to improve the manufacturability of processing, composites containing a mixture of boron fiber with glass fiber are used.
Boron fibers are used in aviation and space technology for the manufacture of various profiles, panels, compressor parts, etc.
Carbon fibers (CFRP) - composite materials based on a polymer binder and carbon fibers. Carbon fibers are characterized by high heat resistance; specific strength, chemical and weather resistance, low coefficient of thermal linear expansion.
Two types of fibers are used: carbonized and graphitized. Viscose or polyacrylonitrile (PAN) fibers, stone and petroleum pitches, which are subjected to special heat treatment, are used as the starting material. In the process of high-temperature processing in a non-oxidizing environment, there is a transition from organic fibers to carbon fibers. Carbonization is carried out at a temperature of 900 ... 2000 °C, and graphitization - at temperatures up to 3000 °C. According to mechanical properties, carbon fibers are divided into high-modulus and high-strength. Thermosetting polymers are used as binders: epoxy, phenol-formaldehyde, epoxy-phenolic resins, polyimides, etc., as well as carbon matrices.
Carbon fibers have good mechanical properties, static and dynamic endurance, water and chemical resistance, etc.
Carbofibers based on epoxy-anilino-formaldehyde binder (KMU-3, KMU-Zl) are operable at temperatures up to 100 °C, on epoxy-phenolic (KMU-1l, KMU-ly) up to 200 °C, on polyimide (KMU- 2, KMU-2l) up to 300 °C, on a carbon matrix up to 450 °C in air and up to 2200 °C in an inert atmosphere.
Carbofibers are used for the manufacture of structural parts for aviation and rocketry, antennas, ships, cars, and sports equipment.
Layered Composite Materials have sheet fillers (fabrics, paper, veneer, etc.), impregnated and bonded together with a polymer binder. These materials have anisotropic properties. As fibrous reinforcing elements, fabrics based on high-strength fibers of various nature are used: cotton, glass-asphalt fabrics, organic fabrics, carbon fabrics, organic glass fabrics, boron-organic glass fabrics. Fabrics differ from each other in the ratio of fibers in the warp and weft, in the type of weave, which affects their mechanical properties. Laminated composites are produced in the form of sheets, pipes, blanks.
Getinaks - plastic based on modified phenolic, amino-formaldehyde and urea resins and various grades of paper.
Organogetinaks is made on the basis of paper from synthetic fibers, most often from aromatic polyamides and polyvinyl alcohol. Polyimides, phenol-formaldehyde, epoxy resins and others are used as binders. Compared with getinaks, they have a higher resistance in aggressive environments and the stability of mechanical and dielectric properties at elevated temperatures.
Textolite - laminated plastic based on polymer binders and cotton fabrics. The material has high mechanical properties, resistance to vibrations. Depending on the main purpose, textolites are divided into structural, electrical, graphite, flexible cushioning.
Structural textolite grades PTK, PT, PTM is used for the manufacture of gears, plain bearings operating at temperatures in the friction zone not higher than 90 ° C, in rolling mills, turbines, pumps, etc. It is produced in the form of sheets with a thickness of 0.5 to 8 mm and plates with a thickness of 8 to 13 mm.
Electrotechnical textolite is used as an electrical insulating material in environments with operating temperatures from minus 65 to +165°C and humidity up to 65%. It is produced in the form of sheets with a thickness of 0.5 to 50 mm grades A, B, G, VCh. Electrical strength in transformer oil up to 8 kV/mm. Grade A - with improved electrical properties for operation in transformer oil and in air at an industrial frequency of 50 Hz. Grade B - with improved electrical properties for operation in air at a frequency of 50 Hz. Grade G - similar to grade A in terms of properties and area of use, but with extended tolerances for warpage and thickness. HF grade - for operation in air at high frequencies (up to 10 6 Hz).
Graphite textolite is used for the manufacture of bearings for rolling equipment and is produced in the form of sheets with a thickness of 1 ... 50 mm, a length of up to 1400 mm and a width of up to 1000 mm.
Flexible gasket textolite is used for the production of sealing and insulating gaskets in machine assemblies exposed to oils, kerosene, gasoline. They are produced in the form of sheets with a thickness of 0.2 ... 3.0 mm.
AT asbestos-textolites and asbogetinaks as fillers, respectively, asbestos fabric or asbestos paper (up to 60%) is contained, and as a binder - phenol-formaldehyde and melamine-formaldehyde resins, silicon-organic polymers, which determine the permissible operating temperature.
Materials based on melamine-formaldehyde allow the operation of products at temperatures up to 200 °C, on phenol-formaldehyde - up to 250 °C and on organosilicon up to 300 °C during long-term operation. For a short time, the temperature can reach 3000 °C. Asbestos-textolites are used mainly for the manufacture of brake pads, brake linings, as heat-insulating and heat-shielding materials.
Glass fiber are made on the basis of glass fabrics and various polymer binders. On phenol-formaldehyde resins (KAST, KAST-V, KAST-R), they are more heat resistant than PTK textolite, but worse in vibration resistance. On organosilicon resins (STK, SK-9F, SK-9A) they have high heat and frost resistance, high chemical resistance, do not cause corrosion of the metal in contact with it. Fiberglass is used mainly for large-sized radio engineering products.
High impact strength KCU up to 600 kJ / m 2, temporary resistance up to 1000 MPa glass fiber anisotropic materials, reinforced with glass veneer (SVAM). In terms of specific rigidity, these materials are not inferior to metals, and in terms of specific strength they are 2 ... 3 times superior to them.
Gas-filled plastics can also be attributed to the class of composites, since their structure is a system consisting of solid and gaseous phases. They are divided into two groups: foam plastics and foam plastics. Styrofoam have a cellular structure, the pores in which are isolated from each other by a polymer layer. Poroplasts have an open porous system and the gaseous or liquid products present in them communicate with each other and the environment.
Styrofoam obtained on the basis of thermoplastic polymers (polystyrene, polyvinyl chloride, polyurethane) and thermosetting resins (phenol-formaldehyde, phenol-rubber, organosilicon, epoxy, urea). To obtain a porous structure, in most cases, gas-forming components are introduced into the polymer binder, called blowing agents. However, there are also self-foaming materials, for example, polyether urethane foam, polyepoxy foam. Foam plastics based on thermoplastic resins are more technologically advanced and flexible, however, the temperature range of their operation is from -60 to +60 °C.
Poroplasts are obtained mainly by mechanical foaming of the compositions, for example, with compressed air or using special foaming agents. During the hardening of the foamed mass, the solvent, being removed from the walls of the cells during the drying and curing process, destroys them. Through pores can be obtained by filling the compositions with water-soluble substances. After pressing and curing the product, it is immersed in heated water, in which soluble substances are washed out.
Foam plastics are used for the manufacture of shock absorbers, soft seats, sponges, filters, as vibration damping and soundproof gaskets in ventilation systems, silencers, gaskets in helmets and helmets, etc. Their density is 25 ... 500 kg / m 3.
Metal-polymer frame materials are composite materials in which the carrier base is a three-dimensional metal mesh, and the interframe cavity is filled with a polymer composition containing various functional components (Fig. 5.11).

Rice. 5.11. The structure of the metal-polymer frame material (a) and the MPC material (b):
1 - metal particles, 2 - polymer, 3 - solid lubricant, 4 - pyrolytic graphite
In mechanical engineering, metal-polymer self-lubricating materials based on a metal-ceramic frame and polymer binders containing various dry lubricants (graphite, molybdenum disulfide, cadmium iodide, etc.) have found application. Such materials are used for the manufacture of plain bearings, rolling bearing cages, piston rings, etc.
To obtain a metal-ceramic frame, powders of tin bronze, stainless steel, glass ceramics are used. Interframe cavities are filled with PTFE-4D by impregnation with a 50% aqueous suspension of PTFE or a mixture of PTFE-4D with lead. Ceramic-metal antifriction material MPK, made on the basis of stainless steel powders, contains pyrographite and fluoroplast-4.
The technology for its production is as follows: metal powders are pressed and sintered into a frame with a porosity of 20 ... 70%. Then, carbon-containing gas is passed through the pores in a special chamber at a temperature that ensures gas pyrolysis and graphite deposition on the frame walls until about 3/4 of the pore volume is filled, after which the product is repeatedly vacuum impregnated with a fluoroplastic-4 suspension with simultaneous heat treatment.
Self-lubricating materials of the given type are efficient at temperatures up to 250 °C.
It is very promising to use tape frame self-lubricating materials, which are a metal base (tape), on which a layer of a porous metal-ceramic frame is baked. The frame pores are filled with compositions based on fluoroplast-4 and solid lubricants.
Tape materials are very technologically advanced, allow the manufacture of plain bearings (rolled) and liners of any size) allow operation without lubrication at temperatures up to 280 ° C at high pressures (up to 200 ... 300 MPa) and sliding speeds. The use of a metal base tape and a bronze porous frame provides good heat removal from the friction zone, and fluoroplast-4 with solid lubricants located in the pores and on the surface ensures a low coefficient of friction and high wear resistance of friction pairs. Abroad, tape materials such as DU, DP, DQ are widely used.
One of the disadvantages of frame tape materials is the small thickness of the surface running-in layer (10 ... 20 µm), which excludes the possibility of machining the bearings after they are mounted in the housing.
It is effective to use frame self-lubricating materials, the frame of which is sintered from metal fibers or meshes, and various polymer compositions are used as a matrix, as well as materials based on carbon-graphite and metallized carbon-graphite fabrics impregnated with polymer binders with solid lubricants.
At present, it is widely used composite wood materials, which are reinforcing wood materials (fillers), combined in a matrix (usually polymeric) with the introduction of special additives. In some cases, they are called wood plastics, or KDPM (composite wood polymer materials).
Particle boards - large-sized products manufactured by hot flat pressing of wood particles mixed with a binder. According to GOST 10632-89, plates are produced in sizes 2440x1220; 2750x1500; 3500x1750; 3660x1830; 5500x2440 mm, thickness from 10 to 25 mm, sanded and not sanded. In accordance with the purpose of the plate are divided into three grades: P-1 (P-1M multi-layer and P-1T three-layer)- manufacture cases, panels and other parts in radio and instrument making, furniture and construction elements. Lined with films based on thermosetting and thermoplastic polymers, paints and varnishes; P-2 (P-2T and P-20 single-layer, subdivided into groups A and B) - manufacture cases of devices, machines, containers and containers (except for food), racks, elements of furniture and building structures. Apply lined with veneer, decorative paper - laminated plastics and without facing; P-3 (P-ET)- body parts for motor vans, car partitions, elements of building load-bearing structures. According to the quality of the surface, the plates are divided into polished (1 and II grades) and unpolished (I and II grades).
Wood fiber boards (GOST 4598-86), depending on the density, they are divided into soft (M), semi-hard (PT), hard (T) and superhard (ST) and, depending on the bending strength, into seven grades: M-4, M- 12, M-20, PT-100, T-350, T-400 and ST-500, where the numbers indicate the minimum value of the ultimate strength of the plates in bending in kgf / cm 2. Plate thickness 2.5; 3.2; four; 5; 6; 8:12; 16 and 25mm, width from 1220 to 1830mm and length from 1200 to 5500mm. Designed for use in products and structures protected from moisture.
Wood laminated plastics (chipboard) - hot-pressed multilayer veneer sheets impregnated with synthetic resins of various types of wood. Chipboards are characterized by high strength and wear resistance, low coefficient of friction and good run-in.
Chipboard from 1 to 15 mm thick are made in the form of rectangular sheets, from 15 to 60 mm thick - in the form of plates. Sheets and slabs glued from whole veneer sheets along the length are called solid, and from several - composite (with somewhat reduced properties). Solid sheets are produced with a width of 950 mm and a length of 700, 1150 and 1500 mm and 1200x1500 mm; composite 2400x950, 4800x1200, 5000x1200 mm; solid slabs: 750x750, 950x700 (1150, 1500); 1200x1200 (1500), compound plates are produced in the same sizes as compound sheets. In accordance with GOST 13913-78 and GOST 20366-75 chipboard is divided into 11 grades.
To the number promising components and parts from KDPM can be attributed:
rollers of belt conveyors;
rolling bearing housings;
blind and passage covers, hatches;
central parts of wheels and rollers (wheel centers with bandages made of steel);
cable blocks for cranes, telphers, chain hoists, etc.;
pulleys, sprockets, gears mounted on shafts with keyless joints;
weights, counterweights, dampers, flywheels with an inner part made of compressed metal shavings and an outer part made of KDPM;
panels for the inner lining of cars, buses, wagons, cabins of various machines, etc.;
pistons of pneumatic and hydraulic cylinders;
window frames;
frames for parts made of polyurethane foam;
bent-glued profiles and veneer panels;
sandwich panels with outer sheets made of plywood, fiberboard, chipboard, DSG1, chipboard or metal (steel, aluminum) and a central part made of foam plastics with wood fillers;
parts made of foam plastics with wood fillers for structural and heat-insulating purposes (for example, fastening parts for ceilings of cars, heat, noise and vibration insulation of cars, diesel locomotives, refrigerators and garage doors, thermal insulation of pipes with channelless laying, etc.);
reservoirs (gas tanks, receivers, etc.).
plain bearings operating in selective transfer mode;
Of course, the considered promising areas of application of the KDPM do not claim to be complete, do not exhaust all possible areas of use, and can be significantly expanded.
In order to correctly understand what this article is about, you first need to correctly define the phrase - thermoplastic composite materials (T.K.M.), and in no case be confused with a compound, since we are talking about completely different materials. So, what is a thermoplastic composite material (composite)? is a heterogeneous multiphase material of two or more components with a clear interface between them and qualitatively new properties while maintaining the chemical identity of each component. It consists of a plastic base (matrix), which serves as a binder, and inclusions of various components in the form of powders, fibers, etc. (filler). The matrix ensures the solidity of the material, the transfer and distribution of stresses between the filler, determines the tightness, heat, moisture, fire and chemical resistance of the composite, its technological, as well as thermophysical, electrical and radio engineering properties. The optimal combination of operational and technological properties is directed to regulate the properties and content of the matrix and filler, interacting between them at the phase boundary, the orientation of the filler. The use of several matrices (polymatrix composites) or fillers of various nature (hybrid composites) expands the possibilities for controlling the properties of composites. Basic grades of polymers are used as a matrix of thermoplastic composite materials. The modern range of basic thermoplastic polymers, depending on the level of their elastic-strength properties and deformation heat resistance, is conditionally divided into three groups.
According to the molecular structure, thermoplastics are divided into two groups - amorphous and crystalline. Due to the structural features, polymers of the second group are of the greatest interest to manufacturers, which can offer a higher level of physical and mechanical properties and greater chemical resistance.
The volume of world production of thermoplastics (in 1990 - 86 million tons, in 2000 - 150 million tons, in 2010, according to forecasts - 258 million tons) significantly exceeds the volume of world production of thermoplastics. As fillers, solid fillers in the form of powders, fibers of various lengths, woven and non-woven structures formed from fibers of various chemical nature can be used. Depending on the functions performed, fillers are divided into three groups:
inert- barite, dolomite, natural chalk, marble, etc. Their use is due to the desire to reduce the cost of the final product, when some deterioration in the properties of the material is acceptable;
Active- mainly based on natural silicates - wollastonite, kaolin, mica, talc. Their improved technological properties are determined “by natural factors: the shape of the particles, the level of their anisotropy, the chemistry of the surface of the particles in relation to polymers;
Functionalized or surface modified. It is known that in order to improve the quality and competitiveness of composite materials, it is important to functionally modify the surface of fillers with organic and/or inorganic compounds, which make it possible to give the filler additional properties that improve or optimize the important parameters of the thermoplastic. It is the third group of fillers that is most promising for the production of thermoplastic composite materials.
In connection with the foregoing, the filler becomes a carrier of special properties, which makes it possible to supplement, replace or save the corresponding technological additives. The use of fillers in polymers allows you to control the properties of products in the widest range of applications.
Thermoplastic composite materials can be conditionally divided into the following groups depending on the required qualities for the final product and scope:
Filled - have increased strength characteristics due to the introduction of mineral fillers - stiffness, strength, shrinkage resistance;
Slow-burning - have increased fire resistance and do not support combustion without an external source of flame due to the introduction of special additives - flame retardants;
Adhesive - have increased adhesive properties in polymer-polymer, polymer-metal systems, etc. by modifying such copolymers as: ethylene vinyl acetate copolymer, ethylene ethyl acrylate copolymer;
Frost-resistant - have increased resistance to low temperatures due to the introduction of mineral fillers and elastomers;
Crosslinked - have increased heat resistance, strength and rigidity due to radiation or chemical crosslinking of the polymer;
Polymatrix - have additional properties that are different from the base grades due to the mixing of different grades of polymers;
Hybrid - have extended options for regulating the properties of the composite due to the introduction of fillers of various nature.
One of the leading problems of modern materials science is the creation of a new generation of thermoplastic composite materials that would satisfy the rather conflicting requirements of manufacturers and consumers.
Dictionary.
Plastics (plastics, plastics)- structural materials containing a polymer, which is in a viscous state during the formation of the product, and in a glassy state during its operation. Depending on the reason for the transition from a viscous to a glassy state that occurs during the molding of products, plastics are divided into thermoplastics and thermoplastics.
Polymers- high-molecular compounds, the molecules of which (macromolecules) consist of a large number of repeating groups, or monomeric units, interconnected by chemical bonds.
Thermoplastics- polymeric materials that allow multiple transition to a viscous state when heated.
thermoplastics, thermosetting plastics- polymeric materials, when heated or under the action of hardeners, turning into an infusible and insoluble state.
Elastomers- polymers and materials based on them. Possessing highly elastic properties in a wide range of temperatures of their operation. Typical elastomers are rubbers and rubbers.
Polymer compounds- compositions based on thermosetting oligomers (epoxy and polyester resins, liquid organosilicon rubbers) or monomers (methacrylates, starting materials for the synthesis of polyurethanes) intended for insulating conductive circuits and parts in electrical, radio engineering and electronic equipment. Basic requirements for compounds: absence of volatile substances; sufficiently high viability; low viscosity.
Heat resistance of polymers- the ability to maintain hardness (that is, not soften) with increasing temperature. The quantitative indicator of heat resistance in these cases is the temperature at which the deformation of the sample under conditions of a constant load does not exceed a certain value.
"PHENOMENOLOGICAL MODEL OF A COMPOSITE MATERIAL BASED ON THE THERMOPLASTIC MATRIX AND SHORT CARBON FIBERS Mashtakov A.P., Melikhov K.V., Manyak..."
PHENOMENOLOGICAL MODEL OF A COMPOSITE MATERIAL BASED ON A THERMOPLASTIC MATRIX AND SHORT CARBON FIBERS
Mashtakov A.P., Melikhov K.V., Manyak I.S.
JSC NPP Radar MMS,
St. Petersburg, Russia
The mechanical characteristics of a composite material consisting of a thermoplastic matrix reinforced with short carbon fibers have been experimentally studied. Characteristics were obtained on specimens cut from injection molded plates from a series of uniaxial tensile tests. The process of injection molding of the plate was modeled by the finite volume method. In this case, the system of equations of motion of the polymer melt as a viscous Newtonian fluid was solved, supplemented by the Folger-Tucker equation for determining the orientation tensors of fibers in the matrix. To build an analytical model of the material, a two-stage homogenization scheme was used: first, the effective characteristics for a single inclusion of a given shape were determined using the Mori-Tanaka scheme, then, based on the calculated components of the orientation tensor, the effective characteristics of the entire cell of a representative volume were determined using the Voit scheme. The fibers were assumed to be elastic isotropic, the matrix was assumed to be elastic-plastic with the Mises criterion and an isotropic, power-law strengthening law (J2-model). As a fracture model, the “first pseudo-grain” fracture model with the Tsai-Hill strength criterion was chosen. The characteristics of the matrix and fibers, as well as the parameters of the failure criterion, were selected iteratively based on the condition of the best agreement between the calculated and experimental strain curves for three types of samples using the least squares method. The presented results in the form of a comparison of the strain curves indicate a satisfactory agreement with the experiment in both the elastic and inelastic regions. REFERENCES
S. T. Chung and T. H. Won. Numerical Simulation of Fiber Orientation in Injection Molding of Short-Fiber-Reinforced Thermoplastics. ENGINEERING AND SCIENCE, MID-APRIL 1995, Vol. 35, NO. 7.-p. 604-618.
B. E. VerWeyst, C. L. Tucker III, P. H. Foss_, J. F. O'Gara. Fiber orientation in 3-D injection molded features: prediction and experiment/ International Polymer Processing, June 18, 1999.
Mori T, Tanaka K. Average stress in matrix and average elastic energy of materials with misfitting inclusions. Acta Metall 1973; 21:571-574.
R. Christensen. Introduction to the mechanics of composites / R. Christensen. – M.: Mir, 1982. – 334 p.
S. Kammoun, I. Doghri, L. Adam, G. Robert, L. Delannay. First pseudo-grain failure model for inelastic composites with misaligned short fibers. Composites: Part A 42 (2011) 1892–1902.
J. M. Kaiser, M. Stommel. Strength prediction of short fiber reinforced polymers. Journal of Plastics Technology 8 (2012) 3, 278-300.
Similar works:
"Contract No. _ for the provision of services for voluntary insurance of a motor vehicle against damage, theft or theft (CASCO), Moscow "" 201_g. Federal State Budgetary Institution of Science Institute ... "
“SUMMARY1. Information about yourself1. Surname Abai2. Name Raushan3. Middle name Madiyarizy4. Date and place of birth 12/01/1994 Karaganda region Karaganda city5. Nationality Kazakh6. Gender Female7. Marital status Not married8. Home address Karaganda, Prikanal ... "
“NOTICE OF CHANGES TO THE NOTICE OF THE ELECTRONIC AUCTION No. 163/А/АВР DATED 01/26/2017 AND TO THE DOCUMENTATION ON THE ELECTRONIC AUCTION PERFORMANCE OF WORKS ON MAJOR REPAIRS OF THE COMMON PROPERTY OF APARTMENT BUILDINGS (repair) and (or) restoration (repair) and (or) restoration (repair) »
"Public Association "Belarusian Karting Federation" CLASSIFICATION AND TECHNICAL REQUIREMENTS FOR RACING CARS "KART" Entered into force on March 1, 2012 Approved by the Council of the BFC Protocol of February 25, 2012 Minsk 20 ... "
""IN THE WORLD OF PROFESSIONS"Goal: formation of a professional orientation of students by expanding their understanding of the construction professions, acquaintance with the programs and initiatives of the President and the Government of the Russian Federation on prestige ... "
“Human Rights Council Thirtieth session Agenda item 5 Human rights bodies and mechanisms Report of the Open-ended Intergovernmental Working Group on the Draft United Nations Declaration on the Rights of Peasants and Other People Working in Rural Areas Chair...”
"Non-profit partnership Self-regulatory organization "Regional Association of Designers" (NP SRO "ROP") Minutes No. 128 of the meeting of the Council of the Self-Regulatory Organization of the Non-Commercial Partnership "Regional Association of Designers" November 22, 2013 Venue ... "
«Roil Platinum, Petrol System Cleaner "Roil Platinum™ Metal Conditioner" Packing: 500 ml., 4 l. Purpose: Over time, your engine wears out from friction. Roil Platinum™ Metal Conditioner can extend the life and power of your vehicle's engine, reduce repair costs by reducing...”
«UDK 621.921 SELECTION OF GRINDING MODES TAKING INTO ACCOUNT TOOL WEAR V.V. Borisov1, I.D. Ibatullin1, D.R. Zagidullina2 1 Samara State Technical University 2 Bashkir State University
"MINISTRY OF EDUCATION AND SCIENCE OF THE RUSSIAN FEDERATION federal state autonomous institution of higher education" NATIONAL RESEARCH TOMSK POLYTECHNICAL UNIVERSITY "APPROVE Deputy. Director of the Institute of Cybernetics for Academic Affairs S.A. Gaivoronsky "_" 2015 WORKING PROGRAM OF THE DISCIPLINE "CONSULTING IN AUTOMATIC ..."
2017 www.site - "Free electronic library - electronic documents"
The materials of this site are posted for review, all rights belong to their authors.
If you do not agree that your material is posted on this site, please write to us, we will remove it within 1-2 business days.
"Definitions and classification of polymer composites Composite materials are materials obtained from two or more components and ..."
-- [ Page 1 ] --
TOPIC 1. DEFINITIONS AND CLASSIFICATION OF POLYMER
COMPOSITES. THE MECHANISM OF INTERACTION OF THE COMPONENTS
The modern era can be called the century of polymers and composite materials.
Definitions and classification of polymer composites
Composite materials are materials made from two or more components and
consisting of two or more phases. One component (matrix) forms a continuous
phase, the other is the filler. Composite materials are heterogeneous systems and can be divided into three main classes:
1. Matrix systems consisting of a continuous phase (matrix) and a dispersed phase (discrete particles).
2. Compositions with fibrous fillers.
3. Compositions having an interpenetrating structure of two or more continuous phases.
Advantages of heterogeneous polymer compositions in comparison with homogeneous polymers:
1. increased rigidity, strength, dimensional stability.
2. increased work of destruction and impact strength.
3. increased heat resistance.
4. reduced gas and vapor permeability.
5. adjustable electrical properties.
6. reduced cost.
It is impossible to achieve a combination of all these properties in one composition. In addition, the achievement of advantages is often accompanied by the appearance of undesirable properties (difficulty in flow, therefore, molding, deterioration of some physical and mechanical properties).
A wide variation in the properties of the compositions can only be achieved by changing the morphology and adhesive strength between the phases.
For uniform transmission of the external action through the matrix and its distribution to all particles of the filler, strong adhesion at the matrix–filler interface is required, which is achieved through adsorption or chemical interaction.
The existence of such bonding between mismatched components in heterogeneous plastics distinguishes them from mechanical blends.
The matrix can be metal, ceramic, carbon. The filler is presented in the form of particles and fibers with significantly higher physical and mechanical properties than the matrix.
The particles are commonly referred to as particulate filler, they have an indefinite, cubic, spherical or scaly shape with sizes from fractions of a mm to micron and nanoscale values.
The inert filler practically does not change the properties of the composition.
The active filler significantly changes the properties of the composition. For example, fibers have elastic-strength characteristics which are two orders of magnitude higher than the properties of the matrix. They can be continuous or short. The diameter of thin fibers is 5-15 microns, thick (boron or silicon carbide) - 60-100 microns. The length of short fibers is from 1-2 to 20-50 mm.
The name of the composites corresponds to the nature of the fibers: glass-, carbon-, organo-, boron-plastics, etc. For hybrid options - glass-carbon plastics, organoboroplasts, etc.
The orientation of the fibers determines the transition from filled plastics to reinforced plastics. It is a system of oriented fibers held together by a polymer matrix. Plastics include materials whose indispensable component is some kind of polymer that is in a plastic or viscous state during the molding of products, and in a glassy or crystalline state during operation. Plastics can be homogeneous or heterogeneous. Plastics are divided into thermoplastics and thermoplastics.
Composite classification:
1. By the nature of the matrix:
thermoset thermoplastic.
hybrid.
Thermosetting matrix - a matrix obtained by curing epoxy, ether, imide, organosilicon and other oligomers in the process of manufacturing composites.
Thermoplastic matrix - a matrix that is melted to impregnate the filler and then cooled. These are PE, PP, polyarylene sulfones, sulfides, ketones.
The hybrid matrix can combine thermoset and thermoplastic components.
2. By the nature and form of the filler.
Organic and inorganic substances of natural or artificial origin. The modulus of elasticity of the filler may be lower or higher than the modulus of elasticity of the binder. Low-modulus fillers, which are usually used as elastomers, without reducing the heat resistance and hardness of the polymer, give the material increased resistance to alternating and impact loads, but increase its coefficient of thermal expansion and reduce deformation resistance. The higher the modulus of elasticity of the filler and the degree of filling, the greater the deformation resistance of the material.
Dispersion-filled composites, Materials based on short and continuous fibers.
The chemical nature of the particles is diverse: chalk, mica, metal oxides, glass spheres, carbon in the form of soot or fullerenes, aerosil, glass or clay flakes, rubber-like inclusions, etc.
Reinforcing fibers - glass, organic, carbon, etc. Highly heat-resistant boron and silicon carbide fibers are also known, which are more often used for reinforcing metals.
3. According to the structure of polymer composites Matrix - for materials based on dispersed and short fibrous particles, Layered (two-dimensional) and volumetric for reinforced plastics based on woven and non-woven materials.
Gradient materials with variable structure.
4. According to the degree of orientation of the filler, the anisotropy of the material:
Composites with a random arrangement of particles and fibers, with an isotropic structure, composites with a unidirectional fiber orientation, with a pronounced anisotropy, 90°), composites with a cross, orthotropic orientation (0, with a given anisotropy, composites with an oblique orientation of fibers at angles different from 90 , composites with a fan structure consisting of layers with different fiber orientations.
5. According to the methods of manufacturing materials and products:
one-stage methods - extrusion and "wet" winding, pultrusion (broaching), vacuum forming, two-stage methods for preliminary production of non-oriented (premixes) or oriented (prepregs) fibrous materials (semi-finished products) impregnated with a binder, followed by molding the material (laminate) by "dry" winding methods , pressing, autoclave molding.
6. By the number of components:
two-component, three-component PCMs combining dispersed particles and short fibers, polyfiber hybrid PCMs combining fibers with similar (glass-organoplastics) or significantly different (glass-carbon plastics) deformability, polymatrix structures, for example, based on a combination of thermosetting and thermoplastic binders.
7. By volume of filler content:
with a non-oriented structure - the content of the filler is 30-40% -, with an oriented structure - 50-75%, highly and extremely filled organo fibers - 75-95% -.
8. By functionality:
single-functional (structural), multifunctional, capable of self-diagnosis (smart), multifunctional, capable of self-diagnosis and self-adaptation (intelligent).
When designing composite plastics, there are two stages (see table):
1-calculation - analytical, 2 - experimental - technological.
1 - includes: analysis of the given loading conditions and determination of a method for constructing plastic with the necessary properties. Representations and formulas taken from the mechanics of composite materials are used:
a) the phenomenological approach is based on the application of the equations of the theory of elasticity, creep, etc. for anisotropic materials, b) - establishing the dependences of the mechanical characteristics of the composition on the size of the filler particles, the mechanical properties of the components, their volume content, etc. These dependences are analyzed at the microscopic, macroscopic and intermediate levels. Microlevel - the level of structural heterogeneity, commensurate with the transverse dimensions of the filler elements - the diameter of the filler particles or the thickness of the reinforcing layer.
Table Required mechanical characteristics of composite plastics Choice of components and their Choice of reinforcement scheme ratio in the composition
– – –
Shape Size ratio Mechanism of PCM components interaction Let us consider the mechanism of stress transfer from the matrix to the filler depending on its configuration.
In the simplest case, when the polymer is reinforced with unidirectional continuous fibers and is stretched in the direction of their orientation, the deformation of the components is the same and the stresses arising in them are proportional to the modulus of elasticity of the fibers and the matrix. If in the same model the fibers are discrete, then the stress distribution turns out to be inhomogeneous along the length of the fiber. There is no tension at the ends of the fiber, but there are tangential stresses at the fiber matrix boundary, which gradually involve the fiber into work. The growth of tensile stresses in the fiber continues until they reach the average level of stresses observed in a continuous fiber. Accordingly, the length at which this occurs is called "inefficient". With increasing strain, the "ineffective" length grows and reaches its maximum value at a stress corresponding to the strength of the fiber. In this case, the “ineffective” length is called “critical” I. It is an important characteristic of the interaction of composites and can be calculated using the Kelly formula lcr/dvol = vol/2mat (1) where dvol and vol are the fiber diameter and strength; mat - matrix yield strength or adhesive strength of the system.
Depending on the strength of the fibers and the type of polymer matrix, the ratio lcr/dvol may vary from 10 to 200; at dvol 10 µm, lcr = 0.15-2.0 mm.
It follows from the above reasoning that in the transition from continuous to discrete fibers, a part of the length of each fiber will not perceive the full load. The shorter the reinforcing fiber, the less effective it is. At l lcr, the matrix under no circumstances can transfer to the fiber a stress sufficient to destroy it. From this it follows that the reinforcing ability of short fibers (an increase in the elastic-strength characteristics of the polymer) is very low. Especially when you consider the orientation of the fibers, which in such materials is not ideal.
The structure of materials based on short fibers is rather chaotic. The advantage of short-fiber fillers is determined by the possibility of high-speed processing of materials into products. However, during the casting or extrusion process, additional destruction of the fibers occurs, the length of which is usually reduced to 0.1-1 mm.
When switching to a dispersed powdered filler, the possibility of stress transfer from the matrix to the filler is so reduced that its contribution to the increase in the strength of the composite begins to compete with the decrease in the strength of the matrix due to the resulting stress unevenness and the development of defects. Because of this, the strength of such a composite usually does not increase compared to the strength of the matrix (sometimes even slightly reduced).
When filling viscous thermoplastics with rigid fillers in an amount of more than 20%, a transition from plastic flow to brittle fracture is observed. In this case, there is a significant decrease in impact strength, work of destruction. The elastic modulus increases with an increase in the amount of filler, but at the same time, the size and number of cracks, “pseudopores” that appear during loading when the matrix is peeled off from dispersed particles at the moment of reaching stresses corresponding to the adhesive strength of the system, increase. Theoretical and experimental studies show that by reducing the size of the filler particles and spreading their diameters, it is possible to significantly reduce the likelihood of large defects.
The main reason for hardening is a change in the direction of crack growth when they come into contact with solid particles of the filler. The most probable direction of crack growth is perpendicular to the direction of the applied force. If a filler particle is located in this direction, then the crack should change its direction tangentially to the surface of the particle. Therefore, if the particles are in the form of fibers and are elongated in the direction of the acting force. Crack propagation along the filler particles is excluded.
When using a monolithic fiber with a round cross section, the mechanical properties usually reach a maximum at 2 = 0.65 - 0.7. When using precision methods for laying profiled fibers, it is possible to increase 2 to 0.85, after which the strength of the compositions begins to depend more on the strength of adhesion at the fiber-binding interface than on the strength of the fiber.
At the same degree of filling (2 = 0.7) and the ratio of elastic moduli (E2/E1 = 21), the rigidity of plastic with triangular fibers in the transverse direction exceeds the rigidity of plastic with round fibers by 1.5 times.
Replacing a monolithic fiber with a hollow one makes it possible to sharply increase the specific strength and rigidity of products in compression and bending, since the moment of inertia increases with the same mass of fibers.
It is inefficient to use hollow fibers in tensile compositions due to the low strength of profiled fibers. When shearing, it is better to use profiled fibers.
Another direction in the creation of particulate-filled polymers is their modification with rubber particles to reduce brittleness and increase impact resistance.
Positive results have been obtained for high impact polystyrene, epoxy and other matrices. The mechanism of hardening of materials is apparently very complex, but the main role is assigned to the inhibition of crack development by rubber particles. Many authors point out the expediency of creating a transition layer with high adhesion to the matrix polymer and the rubber phase in order to increase the strength.
Let us return to a unidirectional composite based on continuous fibers and consider micromechanical models of its destruction. Elementary fibers have very high strength characteristics, ten times greater than the strength of bulk samples. For example, the strength of bulk glass is 50-70 MPa, and in the form of fibers - 2.5-3.0 GPa; a similar picture is observed for organic and carbon fibers, the strength of which reaches 4–6 GPa. This difference is explained either by the influence of the scale factor (the size of the fiber surface determines the size of a possible defect) or by the orientation effect, which is very characteristic of organic fibers.
When testing elementary fibers, a large scatter of experimental strength values is observed. Therefore, at least 50 samples are usually tested, the average value and its variance are found.
Based on the weak link hypothesis, Weibull obtained the following equation for the probability of destruction Р() of a sample under stress and sample length L:
Р() = 1 – exp(–L), (2)
whose constants are determined from the experimentally obtained strength distribution of elementary fibers. The parameter P characterizes the defectiveness of the samples.
Coefficient values vary from 3-5 for normal to 10-12 for "intact" glass fibers.
In reality, one rarely deals with an elementary fiber, usually with a bundle consisting of many fibers. According to the theoretical concepts of Daniels, the decrease in the strength of a bundle of unbonded fibers compared to the average strength of oxen is determined by the dispersion of their strength. In the process of loading, when the tensile strength of any fiber is reached, it breaks and no longer participates in the work.
The force is redistributed to whole fibers, the process continues until the moment of an avalanche-like destruction of the majority, and then all the fibers in the thread (bundle). At =10, the strength of the thread n is approximately 80% of the average strength of the elementary fiber.
Analysis of the thread loading diagram - makes it possible to trace the entire process of gradual fiber rupture. It also makes it possible to identify some defects in the thread, in particular, the difference in length (different tension) of the fibers, which enhances the non-simultaneity of their destruction. Interaction (bondage) of fibers, due to twist or partial bonding, manifests itself in the nature of the diagrams
– which become more linear. The Weibull coefficient for an unbonded bundle of fibers should remain the same as for elementary fibers: In the case of their bonding, it tends to increase.
The polymer matrix that binds the beam into a single whole - microplastic - leads to an increase in its strength. In this case, the strength practically does not depend on the sample length (= 30–50), which indicates a change in the fracture mechanism. The fact is that a fiber torn in some place does not cease to perceive the load, as in a thread, but continues to work at the same level of stress as in neighboring fibers. This occurs at a distance lcr from the fracture site in accordance with the mechanism that was considered above for materials based on short fibers.
According to the statistical theory of strength developed by Gurland and Rosen, the tensile failure of a unidirectional composite occurs through the accumulation of ruptures, crushing fibers in the polymer matrix. In this case, the theoretical strength of the fibers tr in the composite is equal to the strength of an unbound bundle of fibers of the "critical" length lcr.
tr = (lkre)–1/ In practice, the process of crushing fibers cannot be completed. It is usually interrupted by the appearance and development of a main crack due to overstresses in the section where the largest number of defects accumulate, or by delamination at the fiber-binder interface. This mechanism allows obtaining the highest strength values, since it is associated with energy dissipation for the formation of large free surfaces. Based on this, when considering the issue of realizing the strength of fibers in a composite, it is advisable to compare the experimental values of wc with the strength tr, which could be in the implementation of the fiber crushing mechanism:
Kp = ox / tr, where Kp is the coefficient of strength realization.
Its real values reach 60-80% for unidirectional glass-, organo- and carbon-reinforced plastics based on superstrong fibers.
A similar approach has also been proposed to study the realization of the strength of glass-reinforced plastics under longitudinal compression.
Currently, two main options for failure mechanisms are being considered:
Destruction due to buckling of fibers on an elastic base;
Stratification of the material from the impact of shear stresses.
The main dependence arising from the consideration of the first fracture model relates the compressive strength of the material tszh with the shear modulus of the matrix Gm and its volume content m:
tszh = Gm / Vm Calculations carried out according to this formula give very high theoretical values of tszh. For example, with a shear modulus Gm = 1-1.5 GPa, typical for epoxy resins, and m = 30%, the compressive strength tszh could be 3-5 GPa, while for real materials it does not exceed 1.5 GPa .
It can be argued that in all cases there is a proportionality between the strength of glass-reinforced plastics under compression of the HOA and shear shear:
tszh =K shift, which indicates that the second mechanism is prevailing. This can be explained by defects in the structure of the samples and the inhomogeneous stress field that occurs during testing. Special methods for the preparation and study of unidirectional fiberglass made it possible to increase the TCF to 2-3 GPa, that is, to a large extent, it was possible to implement the mechanism of fiber buckling, increasing the strength realization coefficient from 30-40 to 60-70%.
When organoplastics are compressed, destruction occurs along a shear plane oriented at an angle of 45° to the fiber axis, which is typical for plastic fibers.
A similar mechanism seems to take place for carbon plastics, although in this case it is combined with a shear element.
The variety of mechanisms of destruction of composites allows us to raise the question of optimizing the properties of the binder. For example, to increase the tensile strength of the material along the fibers, it is necessary to reduce the "critical" length, which is achieved by increasing the rigidity of the matrix. On the other hand, this leads to an increase in stress concentration and growth of the main crack. The competition of these mechanisms is observed in the form of an extreme dependence of the strength of the composite on the yield strength of the binder, which is varied by changing the temperature, testing speed, or the introduction of plasticizing additives.
In each case, the optimum is:
it depends on the nature of the fibers, the presence of existing technological stresses and defects. The contradictory requirements for the binder are aggravated when taking into account its manufacturability, heat resistance, ability to absorb dynamic effects (impact strength), etc. The weakest point of composite materials is their low strength and shear deformability. Therefore, technological and operational stresses often lead to material cracking.
The crack resistance of a composite is usually characterized by the specific fracture toughness Gc, i.e., the energy dissipated during the formation of a new surface. The higher the specific fracture toughness, the higher the resistance of the composite to delamination. Interlaminar viscosity increases with increasing matrix deformability, fiber-to-matrix adhesion, and fiber-to-fiber bond (VCB) thickness.
The modification of epoxy matrices with rubbers did not lead to a significant improvement in the properties of the materials. Perhaps this is due to the fact that the plasticity zone in the composite is limited by the size of the interfiber space. A much greater effect is observed when using thermoplastic matrices, for example, PSF, the deformability of which reaches 80-100%. In this case, the values of Gc increase by almost an order of magnitude.
Micromechanical models of polymer composites make it possible to reveal analytical dependences showing the influence of the properties of fibers, matrix, their adhesive interaction, material structure, and fracture mechanisms on the macroscopic elastic-strength characteristics of a unidirectional layer. They most successfully describe the limiting modulus of elasticity and tensile strength of the composite. In the case when the deformations of the fibers and the matrix are the same, the following additive relations take place, which show the contribution of each component in proportion to its volume content Ek = Evv + Emm
– – –
These equations are called the "mixture rule".
Since the contribution of the polymer matrix usually does not exceed 2–5%, it can be ignored:
Ек () = Евв and к ()= вв The elongation of the composite under tension in the transverse direction is composed of the deformation of the fibers and the binder. The modulus of elasticity E() can be calculated by the formula 1/ Eк() = v/Ev + m/Em. It should be taken into account that the modulus of elasticity of the fibers themselves in the transverse direction coincides with the modulus of elasticity in the longitudinal direction only for isotropic glass and boron fibers. For carbon and organic fibers, the transverse modulus is significantly lower than the longitudinal one. A similar dependence takes place for the shear modulus of a unidirectional composite "in the plane" of the fibers.
The strength of composites in transverse tension-compression and shear depends on many factors, primarily on the properties of the matrix, adhesive interaction, material structure - the presence of pores and other defects. Analytical dependencies in this case can only have a correlation character. It is generally accepted that reinforcement reduces the strength of the composite in the transverse (transversal) direction by about 2 times compared with the strength of a homogeneous matrix.
Elastic strength properties of composites Strength and stiffness are the most important characteristics of any material. When a sample is loaded by tension or compression, normal stresses and corresponding strains arise in it, which increase until the material is destroyed.
The ultimate (maximum) stress is called its strength. For linear elastic materials, there is a direct proportionality between stress and strain Hooke's law = E. The proportionality coefficient characterizes the stiffness of the material and is denoted as the modulus of elasticity, or Young's modulus E.
This law is also fulfilled when the sample is loaded with shear (tangential) stresses and deformations that occur, for example, during torsion.
The coefficient of proportionality in this case is called the shear modulus G: =.G.
When a material is stretched, simultaneously with elongation, its transverse dimensions are reduced, which is characterized by Poisson's ratio, which establishes a relationship between strains along x and across y of the sample: x = µ y.
The elastic properties of isotropic materials are well described by two constants E and G, the relationship between which corresponds to the equation G = E/2(l + µ).
The above relations well describe isotropic materials, the properties of which are the same in all directions. These include particulate-filled polymers, as well as composites based on short or continuous fibers of a chaotic structure. (For fibrous materials, there is always a certain degree of orientation, determined by the influence of technological factors.) When loading any structure, the stress-strain state of the material most often becomes inhomogeneous. In this case, it is possible to identify the main (maximum) stresses that can cause its destruction. For example, in the case of a pipe under internal or external pressure, the hoop stresses are twice the axial stresses, that is, half the thickness of an isotropic material is ineffective in terms of axial stresses. The inhomogeneity of the stress field can also be significantly higher. For shells with an open exit (guns, grenade launcher barrels), the ratio of radial and axial stresses reaches 8-10 or more. In these cases, one should take advantage of the remarkable ability of fibrous materials, which can be oriented in the matrix in accordance with the distribution of the main service stresses.
Consider the example of a unidirectional layer. The unidirectional layer is isotropic in the direction perpendicular to the fiber orientation axis x. one.
– – –
The tensile strength of the unidirectional layer along the fibers can range from 1.0 to 2.5 GPa depending on the strength level of the fibers, the type and content of the binder. In this case, the strength in the transverse direction does not exceed 50-80 MPa, i.e. the anisotropy coefficient is 20-30.
A slight deviation of the direction of the load from the direction of orientation of the fibers has almost no effect on the tensile strength of the composite. Therefore, some misorientation of the fibers (3-5°) is allowed, created by a special spreader or an increase in the winding pitch in order to increase the transverse strength of the material. In the case of compression, this is unacceptable, since it contributes to the development of shear stresses that determine the compressive strength of the material.
A unidirectional composite is the basis of a complex structure that is created by combining individual layers in accordance with the performance requirements of the structural element. Manufacturing methods: vacuum or autoclave molding, pressing, winding.
Let us further consider theoretical models for describing the processes of deformation and destruction of layered composites of a complex structure. Conventionally, two main approaches can be distinguished in the development of calculation methods: phenomenological and structural. In the phenomenological approach, a composite material is considered as a homogeneous anisotropic medium, the model of which is based on experimentally obtained data. The selected strength criterion applies to the entire material as a whole. The advantage of phenomenological models is the simplicity of calculations. However, for materials with a complex reinforcement scheme, it is required to determine many empirical coefficients, which requires a large number of experiments. In addition, phenomenological models do not take into account structural processes during fracture: cracking, microbuckling, etc.
Determining the optimal size of the filler particles The stress that occurs on different parts of the surface of the particles (microflakes or microfibers) depends on the distance r from the corresponding surface area = – о(1 –)/ 2r, where is Poisson's ratio.
Strength with an increase in the specific surface of the finely dispersed filler increases to a certain maximum, depending on the nature of the components of the composition.
The optimal diameter d of continuous fibers in a stretchable orthotropic plastic at a given distance between the fibers is determined by the equation d (1/2 - 1), where 1, 2 are the relative elongations at break of the binder and filler fibers, respectively.
The choice of filler particle shape The shape of the particles affects the mechanism of plastic degradation. The size and shape of products, processing technology are taken into account.
In the case of products of small thickness and complex configuration, preference is given to highly dispersed fillers (powders), since they are easily distributed in the binder, maintaining the original distribution during the molding of the product.
The use of highly dispersed fillers reduces the likelihood of destruction, delamination of products during subsequent machining.
Solid inclusions in the stretched sample reduce the stress in the contact zone of the binder with the filler, but in the spherical particle itself, the stress exceeds
1.5 times the voltage in the binder zones remote from it, i.e. filler takes the bulk of the load.
The influence of the filler increases if the particles have an ellipsoidal shape and are oriented in the direction of the deformation axis.
Selection of components with the optimal ratio of mechanical characteristics Conditions: adhesive interaction is greater than the cohesion of the binder, both components work together until destruction, ideally elastic behavior of the filler and binder material.
Determining the optimal degree of filling Even reinforcing fibers do not always have a reinforcing effect on plastics. If the ratio of the deformation characteristics of the binder and reinforcing in a unidirectional plastic satisfies the condition св, then up to the critical volumetric content of fibers (в, cr), even a linear decrease in tensile strength = с(1 – в) is observed.
Due to the slight deformation of the binder at break, equal to c, the stress perceived by the fibers is too small to compensate for the decrease in the strength of the polymer matrix. Only starting from v, cr, the total strength of the reinforced fiber can compensate for the decrease in the strength of the matrix, and the strength of the plastic begins to increase.
Each plastic is characterized by its own b, kr, which for the selected polymer binder is the smaller, the stronger the reinforcing fibers, and with the selected type of fibers, it increases with an increase in the strength of the binder c.
The maximum degree of filling v,max ideally corresponds to such a packing density of the fibers at which they touch each other along the generatrices of cylindrical surfaces. The maximum packing density is achieved at different degrees of filling.
OOO w,max = 0.785, hexagonal OOO w,max= 0.907 Tetragonal OOO w,max = 0.907 Tetragonal OOO w,max = 0.907 Tetragonal OOO w,max = 0.924.
The optimal degree is less than the maximum one in,opt 0.846/(1 + min/D)2, where min is the minimum possible distance between the fibers.
Features of the structure and properties of polymer composite materials (PCM).
PCM with a high fiber content. The physico-mechanical properties of composites essentially depend on the relative content of the components. According to the “mixture rule”, the higher the content of fibers, the higher the density of their packing, the higher (ceteris paribus) should be the elastic modulus and strength of the composites. The calculation of the mass content of ox fibers in the material is based on their quantity in the sample, which is determined from technological considerations (linear density, number of fabric layers or winding parameters). For fiberglass, you can use the binder burning method. There is a ratio ox + sv = 1.
Theoretically, the maximum possible content of fibers of the same diameter with the densest hexagonal packing is 90.8% by volume. Taking into account the real dispersion of fiber diameters (10%), this value decreases to approximately 83%. In many studies, the fiber content vol = 0.65 is considered optimal. This value, apparently, characterizes not the thickness of the binder films (they are different), but the fibrous skeleton formed during the formation of the material by one method or another. The impact of force factors (tension during winding and pressing pressure) in this case is ineffective, since it will only lead to the destruction of the fibers.
The real way to increase the elastic-strength properties of composites by increasing the content of fibers is to compact their stacking in the prepreg until their position in the composite structure is fixed. By reducing the viscosity of the binder and increasing the impact of force factors, it was possible to increase the content of glass and organic fibers in a unidirectional composite up to 78% by volume. At the same time, its elastic-strength characteristics increased accordingly. Theoretically, the content of fibers does not depend on their diameter, but in practice this is of great importance. In the case of carbon fibers having a diameter two times smaller compared to glass or organic fibers, it was possible to increase their content in carbon fiber only to 65%, since it is more difficult to overcome friction in such a system and remove excess binder.
When using CBM organic fibers, it is possible to obtain highly reinforced organoplastics with a fiber content of up to 90-95%. This is achieved due to the irreversible thermal deformation of the fibers in a direction perpendicular to their axis, leading to a change in the cross section of the fibers from a round to a cross section of an arbitrary shape due to contact with neighboring fibers. The interaction between the SVM fibers is achieved either through the thinnest layers of the binder, probably partially located inside the fibers, or by autohesive bonding formed during the mutual diffusion of the fiber components.
The elastic modulus and strength of the ring specimens change linearly in almost the entire range of increase in the volume content of fibers, which indicates that the "rule of mixtures" is fulfilled.
The effect of increasing the elastic-strength characteristics of the composite (20-40%) is so significant that it significantly offsets the decrease in shear and transverse properties of materials observed in some cases, as well as an increase in their water absorption.
Highly and extremely reinforced composites should be used in elements that do not experience shear loads. To improve weather resistance, the outer layers of the structure can be made from composites with a normal or high binder content.
HYBRID AND GRADIENT REINFORCED PLASTICS (HAP) WITH
ADJUSTABLE MECHANICAL PROPERTIES
The creation of hybrid polymer composite materials combining two or more types of fibers - glass, organic, carbon and boron - is a promising direction in the development of modern technology, since it expands the possibility of creating materials with desired properties. The most significant factor influencing the nature of the mechanical behavior of HAP, especially in tension, is the magnitude of the limiting strains of the fibers reinforcing the material. Among the HAPs, which combine fibers with similar deformation characteristics, are organo-glass-reinforced plastics and carbon fiber-reinforced plastics.The mechanical behavior of such materials in tension, compression, bending and shear basically follows the additivity principle, i.e. the "rule of mixtures".
A different nature of the regularities is observed in the study of HAP, combining fibers with different deformability. When stretching carbon-glass-, carbon-organic, boron-glass- and boron-organoplastics, the destruction of the fibers does not occur simultaneously.
The limiting deformation of the composite is determined in this case mainly by the deformation of those fibers, the volume content of which prevails.
Let us designate high-modulus fibers by index "1", low-modulus fibers by index "2".
With a high content of fibers with a high modulus of elasticity (and a low limiting strain 1), the strength of the composite is calculated by the formula k1 = 1(ECBb + E11 + E22) With a high content of fibers with a low elastic modulus, the strength of the composite is calculated by the formula + E22) The mechanism of destruction of three-component materials changes upon reaching a certain critical ratio of fibers of different modulus µcr, at which the destruction of fibers with different breaking elongations is equally probable, i.e. k1 =.
k2. Neglecting the strength of the matrix, we obtain the ratio 1 E11 + 1E22 = 2 E22 after the transformation of which we have:
1/ 2 = k = E2(2 – 1)/ 1 E1 Since 2 = 1 – 1, then µkr2 = k/(1 + k).
For carbon fiber reinforced plastics, one can take E1 = 250 GPa, E2 = 95 GPa, 1=0.8%, 2=3.5%, then k=0.3; µcr1 = 23% or µcr2 = 77%.
The concept of critical volume also takes place for composites based on one type of fiber. It characterizes the transition from the destruction of the binder to the destruction of the fibers.
Due to the large difference in their elastic characteristics, µcr is very small and amounts to 0.1-0.5% of the fibers.
Let us consider the deformation curves of carbon fiber reinforced plastics with different content of dies of different modulus. In the initial section I, the deformation curves are linear, carbon and glass fibers are deformed together, the elastic modulus is composed of two components and corresponds to additive representations. Samples containing more than the critical amount of carbon fibers are destroyed at a deformation of 0.7-0.9%. Nonlinear section II on the deformation curves of carbon fiber reinforced plastics, in which the content of carbon fibers is less than the critical one, can be considered as a “pseudoplasticity” section due to the gradual crushing of carbon fibers in the fiberglass matrix, which ensures the integrity of the material. Nonlinear section II ends at a strain of approximately 2%. Next, an almost linear section III is observed, in which the elastic modulus corresponds to the proportion of glass fibers in the composite, and the ultimate strain
– ultimate strain of glass fibers 2 3-3.5%.
When the sample is reloaded, the diagram is completely linear and corresponds to the third section of the original curve. At the same time, fiber fragmentation apparently occurs during another two or three load-unload cycles, since only after this is a constant correlation dependence of the electrical resistance on the deformation of the sample established.
The dependence of the tensile strength of HAP on the ratio of fibers of different modulus is characterized by a curve with a minimum corresponding to the critical ratio of fibers.
For materials tested in compression, the diagrams - and strength dependences are almost linear. Low-strength (in compression) organic and carbon fibers, being in a glass- or boron-plastic matrix, may not lose stability during deformations and, therefore, at stresses 2-3 times greater than in conventional organic and carbon plastics. These effects, as well as an increase in the deformability of carbon fibers in a fiberglass matrix under tension, are called synergistic by many authors.
Fibers of different types are mixed within one layer or alternate layers.
Below are a few examples of the most rational combination of fibers of different modulus in HAP:
the combination of glass and organic fibers makes it possible to obtain materials, on the one hand, with higher compressive and shear strength (compared to organoplastics), on the other hand, to increase the specific tensile characteristics of the hybrid system (compared to fiberglass);
HAP based on a combination of glass and carbon fibers have a higher modulus of elasticity compared to fiberglass, while maintaining the specific characteristics of the strength of materials in compression and slightly decrease in tension; the work of destruction of samples increases;
The addition of boron fibers to glass-reinforced plastics can significantly increase their modulus of elasticity, while maintaining (or increasing) the compressive strength of materials.
One of the varieties of HAP is gradient PCM, the structure and properties of which are spatially inhomogeneous. A smooth, controlled change in the elastic-strength properties of PCM in some cases makes it possible to create a uniform stress field. For example, when loading homogeneous PCM shells with internal or external pressure, with an increase in the thickness of the structure, a significant decrease in their effective elastic-strength characteristics is observed. Only the layers adjacent to the pressure medium are fully loaded. Starting from a certain thickness, PCM practically ceases to take additional load, and increasing the shell thickness does not make sense. Theoretically, this phenomenon can be avoided if PCM with a variable (increasing in thickness) modulus of elasticity is used.
In this case, the weight and size characteristics of the material will be improved by 1.5-2 times.
In practice, this option can be implemented, for example, by winding a PCM shell in layers, gradually (according to the calculation) increasing the amount of carbon fibers relative to glass ones. Similar problems (and their solutions) are also encountered in the creation of super flywheels or rotor shrouds rotating at high speed. Varying the position of layers with different fiber content makes it possible to increase the shear, vibration and fatigue strength, water and weather resistance of materials.
Gradient-structural composites significantly expand the possibilities of PCM.
Almost all "natural structures" have such a structure (trunks and stems of plants, protective needles of plants and animals, beaks and feathers of birds, and many other examples). It is obvious that in this matter there is a strong lag behind nature and there is a huge reserve for improving the performance of artificially created products.
"Intellectual" composites At the end of the XX century. in materials science, a new term has appeared - "intelligent"
materials. The accepted concept of "intelligent" material defines it as a structural material capable of self-diagnosis and self-adaptation. These materials must be able to recognize the emerging situation (sensory function), analyze it and make a decision (processor function), as well as excite and carry out the necessary reaction (executive function).
Currently, there are no composites that would meet all of these requirements. However, these tasks can be partially (step by step) solved, first of all - the tasks of creating materials that inform about their state, about approaching operational loads to the maximum allowable, about cracking, chemical corrosion, water absorption, etc.
The main requirement for the sensor elements of such composites is sensitivity to mechanical stress and the ability to be distributed throughout the volume. An ideal sensor would convert strain into electrical signals. In this sense, conductive fibers are promising, which can be introduced into composites during their formation. These include constantan or nichrome wire, conductive carbon or boron fibers, piezoelectric films made of polyvinylidene fluoride, etc.
The control of viscoelastic properties of polymer composites (defectoscopy) is carried out using acoustic methods, fixing the relationship between the speed of sound and its absorption coefficient. When using the magneto-dielectric properties of polymers for PCM diagnostics, it is recommended to add dispersed (colloidal) particles of magnetic and electrically conductive materials, including ultrafine powders of iron, copper, nickel, carbon nanoparticles (fullerenes and nanotubes).
The operating principle of the actuating (adaptive) mechanisms is the deformation created as a result of any phenomena - heating, supplying an electrical signal, etc. The piezoelectric effect, electro- and magnetostriction, and the shape memory effect are most suitable for activating the material. These mechanisms ensure that the electrical signal is converted into a triggered strain. The greatest effect is observed for metals with shape memory. An alloy of titanium and nickel provides deformation up to 2%. Another important indicator of the actuator is its modulus of elasticity, which determines the possibility of creating a given stress-strain state. It is usually comparable to the modulus of elasticity of the base material.
The manufacturing process of "intelligent" composites basically corresponds to the process of obtaining a product from the base material. In this case, it is necessary to introduce information and executive elements into the material, minimally violating its structure. It is also necessary to pay attention to the complexity of the micromechanical processes that occur during the curing of the binder.
“Intelligent” composites are, of course, the material of the future, however, intensive scientific and technical work is already being carried out abroad (in the USA, Japan, Great Britain, Canada) to create such materials for modern technology, primarily aviation, rocket and space, etc. etc., as well as for the mass media. Examples of designs that use "smart" materials include the leading edge of the F-15 wing, the segmental reflector and the actuators of the turn structure of spacecraft, aircraft with reduced noise and vibration. German firms that create modern wind power generators monitor the condition of blades with a diameter of up to 100 m or more. Optical fibers placed inside the material allow to monitor its structural integrity and evaluate the loads acting on the blades in order to automatically maintain them at an optimal level. The possibility of delamination of the material, for example, due to a lightning strike, is also controlled.
Dependence of the properties of composite plastics on the interaction of components The mutual influence of the components in the interfacial zone is determined by the composition of the composition and the conditions for its formation. In rare cases, it is possible to establish a functional relationship between mechanical characteristics and interaction.
When sizing increases adhesive strength, there is a correlation between adhesive strength and tensile stress.
The choice of fiber arrangement is based on data on the distribution of the force field and the nature of the loading.
Residual stresses in products made of composite materials affect the performance properties. Residual stress (mechanical, thermal, shrinkage, diffusion, etc.) is understood as stresses that are mutually balanced in the volume of the product, appeared in it as a result of exposure to external force, thermal, and other fields and exist in the product after the termination of the field and the disappearance of temporary stresses. Temporary temperature, shrinkage, diffusion stresses disappear as soon as the temperature, the depth of curing, the degree of crystallinity or the amount of absorbed substance are the same throughout the volume of the material. Mechanical temporary stresses disappear after the termination of the external field.
Residual stresses arise in the molded product only when the maximum temporary stresses in some part of the product volume exceed the yield strength of the material and deformations that are irreversible at ordinary temperature (plastic and highly elastic) occur in it, or due to an unequal degree of transformation (hardening, crystallization ) separate areas of the volume of the material will acquire different thermoelastic properties. The difference in the thermoelastic properties of the polymer matrix and filler also leads to the appearance of residual stresses.
The molding process is carried out at elevated temperatures and pressures.
Consequently, temperature gradients occur which increase even more, since curing usually proceeds exothermically.
During cooling, significant thermal stresses arise in the surface layers, which can lead to additional irreversible deformations and cause an increase in residual stresses in finished products.
Method for determining residual stresses. solvent method.
The sample is treated with a solvent that penetrates the polymer and increases the tension of the surface layer. When the surface stress exceeds the breaking stress of the swelling layer, a network of small cracks will appear in it. In this case, lg = lgm + nlgores, where res is the residual stress (kg/cm2), m and n are constant values.
Stress at the interface between the binder and the filler.
The main reason is the shrinkage of the polymer matrix during curing and cooling, which differs significantly from the temperature shrinkage of the filler bonded to the matrix by an adhesive bond. The pressure of the cured resin on the filler can be calculated by the equation (1 2)TE 2 P=, (1 + 1) + (1 + 2)(E1 / E 2) where 1 and 2 are thermal expansion coefficients, T is the difference between curing temperatures and cooling, 1 and 2 - Poisson's ratios, E1 and E2 - deformation moduli (1 - binder, 2 - filler).
If the stresses that occur in the material are not symmetrical, they can cause shape distortion.
TOPIC 2. UNSATURATED POLYESTER RESINS
Unsaturated oligoesters are called oligomeric esters obtained using unsaturated monomers containing a vinyl group. Such oligomers are widely used in the production of reinforced plastics and other composite materials. In this case, unsaturated oligoesters of two types are used: oligoethermaleinates and oligoetheracrylates.The idea of a combination of reactive polymers and monomers was proposed by C. Ellis in the 1930s, who discovered that unsaturated polyester resins obtained by reacting glycols with maleic anhydride cured into an insoluble solid material when a peroxide initiator was added. Ellis patented this discovery in 1936.
Oligoether maleinates are produced by the interaction of maleic anhydride with dihydric alcohols (ethylene glycol, diethylene glycol, 1,2-propylene glycol), while in order to control the number of double bonds in the resulting oligomer and obtain the final polymer with the required properties, other dicarboxylic acids (adipic, isophthalic, phthalic anhydride, etc.). It should be noted that during the synthesis of oligomers, which is carried out when heated from 50 to 230 ° C, there is a partial or almost complete isomerization of maleate units into fumarate ones: High Quality.
Ellis later discovered that more valuable products could be obtained by reacting an unsaturated polyester alkyd resin with monomers such as vinyl acetate or styrene. The introduction of monomers significantly reduces the viscosity of the resin, which makes it easier to add initiator to the system and allows the curing process to be more vigorous and complete. In this case, the polymerization of the mixture is faster than each component separately.
Since curing proceeds by a radical mechanism, initiators are introduced into the mixture during curing, serving as a source of free radicals and initiating a polymerization chain reaction. Free radicals can be generated from peroxides or other unstable compounds such as azo compounds. To increase the rate of their decomposition, activators (promoters) are additionally introduced into the composition. Typical curing initiators are benzoyl hydroperoxide and cumene hydroperoxide. acids. Co naphthenate is usually used to cure polymaleate styrene binders at 20–60°C. At 80 - 160 ° C - benzoyl peroxide, dicumyl.
Oxygen is an inhibitor. Therefore, waxy substances are introduced. Possessing a low softening temperature and being a surfactant, they cover the surface of the binder and protect it from oxygen access.
Sometimes flame retardants are introduced into polymaleate binders to increase fire resistance: Sb2O3, chlorine- and phosphorus-containing organic compounds.
Styrene-free polyester compositions are obtained by replacing styrene with less volatile (styrene is volatile and toxic) monomers, such as divinyl benzoate, vinyl toluene, diallyl phthalate.
Instead of styrene, triethylene glycol dimethacrylate (THM-3) is successfully used as an active diluent:
At room temperature, liquid resins are stable for many months and even years, but with the addition of a peroxide initiator, they solidify in a few minutes. Curing occurs as a result of the addition reaction and the transformation of double bonds into simple ones; it does not form any by-products. Styrene is most commonly used as the addition monomer. It interacts with the reactive double bonds of polymer chains, crosslinking them into a strong three-dimensional structure. The curing reaction takes place with the release of heat, which in turn contributes to a more complete process. It has been found that usually about 90% of the double bonds present in the polymer enter into the reaction during the curing of the resin.
Oligoetheracrylates are obtained by polycondensation of polyhydric alcohols, saturated aliphatic dicarboxylic acids and unsaturated aliphatic acids of the acrylic series. For the synthesis of these oligomers, dihydric alcohols (glycols) are usually used. Oligoetheracrylates are liquid or low-melting substances with a molecular weight of 300-5000. Polymerizing in the presence of radical polymerization initiators, they turn into infusible and insoluble polymers of a three-dimensional structure, which, depending on the chemical structure of the initial oligomer, are solid glassy or elastic materials. Oligoetheracrylates are capable of copolymerization with various monomers (styrene, methyl methacrylate, etc.), as well as with polyethermaleinates.
Oligoetheracrylates have a certain advantage over oligoethermaleinates: they are capable of homopolymerization, which makes it possible to prepare varnishes and other compositions based on them without the use of volatile and toxic unsaturated monomers.
In the art, oligoetheracrylates are cured by radical polymerization or copolymerization; volumetric shrinkage during curing is 4-10%.
Curing initiators at 50-120 °C (hot curing) are benzoyl, dicumyl peroxides, etc. For curing at room temperature (cold curing), binary systems are used (for example, benzoyl peroxide + dimethylaniline; cumene hydroperoxide + naphthenate or cobalt linoleate).
Curing of oligoetheracrylates can also be initiated by light, high energy radiation (-rays, fast electrons) and ionic polymerization catalysts.
Epoxyacrylate oligomers can be considered as a kind of oligoetheracrylates. Obtained by the interaction of oligomers containing terminal epoxy groups with methacrylic or acrylic acids.
Allyl alcohol ester prepolymers are prepared by polymerization of allyl alcohol esters and phthalic or isophthalic acids. Less commonly used are diallyl maleinate, diethylene glycol-bis-allyl carbonate or triallyl cyanurate.
Polymerization is carried out in a monomer medium by precipitating the prepolymer with methanol, or in a thin layer of monomer with distillation of its excess at a given stage of the reaction in a vacuum.
The reaction is stopped before gelation begins, i.e. up to 25% conversion of all double bonds in the monomer. Molecular weight 6000, softening point ~60o C.
The prepolymers have a long pot life at n.o. and high cure rate at 135-160°C in the presence of dicumyl peroxide or tert-butylperbenzoate. Prepolymers are more often used in the production of prepregs and premixes that have a reduced viscosity and fill molds at low pressure.
Polyester resins are used in a wide range of products including boats, building panels, automotive and aircraft parts, fishing rods and golf clubs. Approximately 80% of polyester resins produced in the USA are used with reinforcing fillers, mainly fiberglass.
Non-reinforced polyester resins are used in the production of buttons, furniture, artificial marble and body putty.
Unlike most other plastics, which consist of a single ingredient, polyester resins often contain multiple components (resin, initiator, filler, and activator). The chemical nature and ratio of components can vary, which allows you to get a large number of different types of polyester resins.
Maleic anhydride is used as a source of reactive double bonds for a large number of unsaturated polyester resins. When it interacts with glycols (usually propylene glycol is used), linear polyester chains with a molecular weight of 1000 ... 3000 are formed. Despite the lower cost of ethylene glycol compared to the cost of propylene glycol, the former is used only to obtain a few special resins. This is due to the poor compatibility of ethylene glycol-based polyesters with styrene. During the esterification process, the cis-configuration of maleic anhydride transforms into the fumaric trans-structure. This is useful due to the greater reactivity of the double bonds of the fumaric fragment in the reaction with styrene. Thus, a high degree of trans isomerization is an important factor in the production of reactive polyester resins. Despite the high degree of maleic anhydride isomerization, which reaches more than 90%, more expensive fumaric acid is used to obtain polyester resins with increased reactivity.
Other diaxial acids or anhydrides, such as adipic and isophthalic acids or phthalic anhydride, are often added to the base reagent to change the final properties of the resin and control the number of double bonds.
A typical polyester resin structure is given below (where R is the alkyl or aryl group of the modifying dibasic acid or anhydride):
O O CH3 O O CH3 II II I II.11 I H [O-C-R-C-O-CH-CH2-O-C-CH=CH-C-O-CH-CH2]nOH low cost polyester resins are widely used to produce various products.
Types of Unsaturated Polyester Resins The wide variety of properties of polyester resins make them suitable for use in a variety of applications. Below is a summary of seven specific types of unsaturated polyester resins.
– – –
This type of polyester resin is obtained by esterification of propylene glycol with a mixture of phthalic and maleic anhydrides. The ratio of phthalic and maleic anhydrides can vary from 2:1 to 1:2. The resulting polyester alkyd resin is mixed with styrene in a ratio of 2:1. Resins of this type have a wide range of applications: they are used for the manufacture of pallets, boats, shower parts, racks, swimming pools and water tanks.
2. Elastic polyester resin
If linear dibasic acids (for example, adipic or sebacic) are used instead of phthalic anhydride, then a much more elastic and soft unsaturated polyester resin is formed. The diethylene or dipropylene glycols used instead of propylene glycol also give the resins elasticity.
The addition of such polyester resins to general purpose rigid resins reduces their brittleness and makes them easier to process. Elastic resins can also be obtained by replacing part of the phthalic anhydride with tall oil monobasic acids, which create flexible groups at the ends of polymer chains. Such resins are often used for decorative molding in the furniture industry and in the manufacture of picture frames. To do this, cellulose fillers (for example, crushed walnut shells) are introduced into elastic resins and cast into silicone rubber molds. Fine reproduction of wood carvings can be achieved by using silicon rubber molds cast directly on the original carvings.
3. Elastic polyester resins Polyester resins of this type are intermediate between rigid general purpose resins and elastic ones. They are used to make impact-resistant products such as balls, crash helmets, fences, automotive and aircraft parts. To obtain such resins, isophthalic acid is used instead of phthalic anhydride. First, by reacting isophthalic acid with glycol, a low acid number polyester resin is obtained. Then add maleic anhydride, and continue the esterification. As a result, polyester chains are obtained with a predominant arrangement of unsaturated fragments at the ends of molecules or between blocks consisting of a glycol-isophthalic polymer. In this type of esterification, phthalic anhydride is much less efficient than isophthalic acid, since the resulting phthalic acid monoester tends to reconvert to the anhydride at the high temperatures used in the production of high molecular weight polyester resins.
4. Polyester resins with low shrinkage
When molding fiberglass reinforced polyester, the difference in shrinkage between resin and fiberglass results in pitting on the surface of the product. The use of low shrinkage polyester resins reduces this effect, and the cast products thus obtained do not require additional sanding before painting, which is an advantage in the manufacture of automotive parts and household electrical appliances.
Polyester resins with low shrinkage include thermoplastic components (polystyrene or polymethyl methacrylate), which are only partially dissolved in the original composition. During curing, accompanied by a change in the phase state of the system, the formation of microvoids occurs, compensating for the usual shrinkage of the polymer resin.
5. Weather resistant polyester resin
This type of polyester resin should not turn yellow when exposed to sunlight, for which ultraviolet (UV) absorbers are added to its composition. Styrene can be replaced by methyl methacrylate, but only partially, because methyl methacrylate does not interact well with the double bonds of fumaric acid, which is part of the polyester resin. Resins of this type are used in the manufacture of coatings, exterior panels and skylights.
6. Chemically resistant polyester resins Ester groups are easily hydrolyzed by alkalis, as a result of which the instability of polyester resins to alkalis is their fundamental disadvantage.
An increase in the carbon skeleton of the original glycol leads to a decrease in the proportion of ester bonds in the resin. Thus, resins containing "bisglycol" (the reaction product of bisphenol A with propylene oxide) or hydrogenated bisphenol A have a significantly lower number of ester bonds than the corresponding general purpose resin. Such resins are used in the manufacture of chemical equipment parts: fume hoods or cabinets, chemical reactors and vessels, and pipelines.
7. Flame retardant polyester resin
Glass fiber reinforced polyester resin moldings and laminates are combustible but have a relatively slow burning rate. An increase in the resistance of the resin to ignition and combustion is achieved by using halogenated dibasic acids instead of phthalic anhydride, for example tetrafluorophthalic, tetrabromophthalic and "chlorendic" (the product of the addition of hexachlorocyclopentadiene to maleic anhydride, which is also known as chaet acid). Dibromoneopentyl glycol may also be used.
A further increase in fire resistance is achieved by introducing various flame retardants into the resin, such as phosphoric acid esters and antimony oxide. Flame retardant polyester resins are used in fume hoods, electrical components, building panels, and the hulls of some types of naval vessels.
The seven types of unsaturated polyester resins described are the most commonly used in the industry. However, there are also resins for special purposes. For example, the use of triallyl isocyanurate instead of styrene significantly improves the heat resistance of resins. By replacing styrene with the less volatile diallyl phthalate or vinyl toluene, monomer loss during polyester resin processing can be reduced. Specialty resins can be cured with UV radiation by incorporating photosensitive agents such as benzoin or its ethers.
Production of unsaturated polyester resins Typically, batch processes are used to produce unsaturated polyester resins. This is due to the variety of starting products needed to obtain different resins, since the periodicity of the process allows a quick and easy transition to the production of other resins. Continuous processes are usually used for large-scale production of general purpose resins.
The preferred construction material for the manufacture of equipment is stainless steel, due to its chemical resistance to polymer resins and other reagents used in the production of polyester resins.
Since iron and copper ions inhibit the free radical polymerization of polyester resins, these materials are not used to make reactors. When using halogen-containing materials as feedstock, glass-lined reactors are preferred.
Usually glycol is loaded into the reactor, and then phthalic and maleic anhydrides are added. Typically, a 5 to 10% excess of glycol is used to compensate for losses due to evaporation and side reactions. Before mixing and heating, the air in the reactor is displaced with an inert gas. The first stage of the reaction - the formation of "half-ester" - occurs spontaneously at a relatively low temperature, after which the reaction mass is heated to complete the formation of the ether. The flow rate of the inert gas through the reactor can be increased to drive off the water generated by the condensation reaction. To more completely remove water from the glycol returned to the reactor, a steam-heated heat exchanger is often used.
During the last stage of esterification, the temperature of the reaction mass rises to 190 - 220 °C. A higher temperature favors the isomerization of maleates to fumarates, but at the same time causes side reactions at double bonds. There is an optimum temperature at which the proportion of fumarate reaches its maximum. For general purpose resins, this occurs at 210°C.
To control the degree of esterification, the acidity and viscosity of the reaction mass are determined, and upon reaching the required values, the polyester is pumped into the final reactor.
The required amount of styrene is already in this reactor, and the polyester alkyd resin is dissolved in it as it arrives. To exclude any polymerization processes that can occur when hot alkyd resin is in contact with styrene, an inhibitor can be additionally added to the reaction mass at this stage. Sometimes, to maintain the required temperature, the reaction mass must be cooled. After the completion of the process, the compliance of the properties of the reaction mass with the technical requirements is checked. A complete production cycle lasts 10 - 20 hours. The described method for the production of polyester resins is often implemented as a melting process. The reactant melt is heated until the conversion reaches the desired level. Another method uses a small amount of solvent (toluene or xylene) to remove the water released during the esterification process as an azeotropic mixture.
The solvent is not more than 8% of the entire reaction mass; it is separated from the water by decantation and returned to the reactor again. After the end of the esterification process, the remaining solvent is distilled off from the reaction mixture, first at atmospheric pressure, and then for its complete removal - under vacuum. During esterification, some side reactions may occur. For example, the glycol hydroxyl group can be added to the double bond of the maleic or fumaric moiety to form a branched polymer. It has been established that about 10 - 15% of the double bonds of the unsaturated polymer are spent on side reactions.
The simplest continuous process for the production of unsaturated polyester resins is the reaction of a mixture of maleic and phthalic anhydrides with propylene oxide.
A small amount of glycol is required to initiate this chain reaction. Since the reaction of anhydrides with epoxy groups occurs at relatively low temperatures, the maleate double bonds do not isomerize into the more active trans configuration. To carry out this isomerization, necessary for further interaction with styrene, the resulting polymer must be subjected to additional heating.
Continuous production of polyester resin from anhydrides and glycols can also be done in a series of heated agitated reactors by sequentially pumping the resin through reactors at different temperatures.
Curing Unsaturated Polyester Resins Unsaturated polyester resins are cured by adding initiators that generate free radicals and initiate the polymerization chain reaction.
Free radicals can be formed from peroxides or other unstable compounds such as azo compounds. These compounds can be cleaved into radical fragments when heated or exposed to ultraviolet or other high energy radiation. Generally, the polyester resin contains an inhibitor which is essentially a free radical scavenger. The polymerization reaction with the introduction of initiators begins only after the action of inhibitors has been overcome. This induction period makes it possible to mechanically mix the resin containing the initiator with the reinforcing agent and place it in the form required for curing before the polymerization reaction begins. Good polymerization inhibitors are hydroquinone and its derivatives, as well as quaternary ammonium halides.
Most peroxide initiators decompose relatively slowly when they enter the polymer mass. To increase the rate of their decomposition, activators (promoters) are used. In fact, activators are catalysts for initiators.
Both the initiator and the activator are reactive compounds, the violent interaction of which is accompanied by ignition or even an explosion. These compounds should be added to the resin separately, making sure before adding the second that the first is completely dissolved. Many resins contain a pre-added activator.
The behavior of polyester resin during curing is determined by the ratio of the influences of the inhibitor, initiator and activator.
Substituents on the ethylene carbon atom can affect the reactivity of the double bond in two ways. Spatial influence is due to the fact that bulky groups shield the double bond and reduce the possibility of the second reactive group to take a favorable position for attack, thereby reducing the reactivity of the entire compound. Polarity is determined by the ability of a substituent group to attract or donate electrons. Electron donating groups (such as methyl, phenyl, and halogen) make the double bond electronegative. It is their action that is manifested in styrene, vinyltoluene and chlorinated styrene.
Electron withdrawing groups (such as vinyl or carbonyl) make the double bond electropositive. This occurs in the fumaric acid fragments in the polyester resin chains. The opposite polarity of the double bond in styrene and fumaric fragments of alkyd resin promotes their interaction and curing of polyester resins. Monomeric styrene, which is more mobile than the long polymeric chains of unsaturated polyester, can be homopolymerized. It has been experimentally established that the molar ratio of styrene and double bonds of polyester 2: 1 is optimal.
initiators and activators
There is a wide variety of initiator-inhibitor-activator systems available for use in the production of polyester resins. For example, a general purpose hydroquinone-inhibited resin can cure very quickly when an active peroxide initiator such as methyl ethyl ketone peroxide is used in combination with an activator such as naphthenate or cobalt octoate. In another case, a much more stable initiator is introduced to cure the polyester resin: tert-butylperbenzoate. This allows the polyester composition to be filled with calcium carbonate and ground fiberglass. This initiator-containing and molded compound is stable at room temperature for months, but can be cured within one minute by hot pressing at 140-160°C.
The choice of a suitable initiator and its amount depends on the type of resin and its curing temperature, the required time for the entire process and the time of gelation. Since none of the available initiators usually satisfies all the necessary requirements by itself, various combinations of initiators and initiators with activators are used to obtain the best results.
In the thermal curing of polyester resins, the most commonly used initiator is benzoyl peroxide (BP), which is extremely effective and easy to use. It is easily soluble in styrene, can be stored for a long time without loss of activity, is stable at room temperature, and easily decomposes at elevated temperature. In addition, BP causes a high exothermic peak temperature, which contributes to the complete curing of the resin. The amount of BP introduced into the resin varies from 0.5 to 2% depending on the type of resin and the monomer used. When using BP in the form of a paste (usually in a mixture with 50% tricresyl phosphate), the amount of the introduced initiator slightly increases (~1 - 3%).
It is sometimes desirable (or even necessary) to carry out the resin curing process from start to finish at low temperatures so that the heat generated during polymerization is dissipated. This is especially important in wet laminating where the use of heat is difficult. In such cases, methyl ethyl ketone peroxide (MEKP) is usually used as the initiator. Although the use of PMEK does not cure the resin completely at room temperature, the addition of an activator (eg cobalt naphthenate) causes the resin to gel and nearly cure within a short period of time.
TOPIC 3. DIESTER-BASED RESINS
VINYL CARBOXY ACID
Resins based on diesters of vinylcarboxylic acids (VCA) are thermosetting polymers, the main chain of which is esterified at the terminal hydroxyl groups with a residue, R, acrylic (I: R=H) or methacrylic (II: R=CH3) acid: -O-C- C-R=CH2. The main chain of macromolecules of these resins are epoxy, polyester, polyurethane or other segments, and practically valuable materials are obtained on the basis of epoxy resins.Although various DVAs have been produced in laboratory quantities since the late 1950s, commercial production of these resins was not established until 1965 by Shell Chemical under the trade name "epocrylic resins". These resins were identified as epoxy methacrylates and had excellent chemical resistance, surpassing that of the best (at the time) polyester resins.
In 1966, Dow Chemical launched Derakan, a diester of vinyl carboxylic acids, and a number of similar coating resins. In 1977, the firms Interplastic and Reichhold Chemical began the production of DVK under the name Coretsin and Korrolit
respectively.
Resin characteristics
Resins can be used both in pure form (i.e. without diluent) or mixed with other ingredients. In the latter case, the resin may contain a reactive vinyl-containing comonomer (styrene, vinyltoluene, trimethylolpropane triacrylate) or a non-reactive "diluent" (methyl ethyl ketone, toluene). As a rule, resins based on esters of methacrylic acid contain styrene and are used in the manufacture of chemically resistant glass fiber reinforced plastics (GRP). Resins - derivatives - of acrylic acid are supplied undiluted, and the corresponding co-reagents are introduced directly during the preparation of coatings and printing inks cured under the action of UV radiation.
The physical properties and applications of DVK depend on the type of end groups (methacrylic or acrylic), on the amount and type of co-reagents, as well as on the nature and molecular weight of the blocks that make up the main chain of resin macromolecules. As a result of curing, styrene - containing DVKM-II acquire high resistance to acids, bases and solvents. Acrylic acid derivatives are more sensitive to hydrolysis than methacrylic acid derivatives, and therefore they are usually not used in the manufacture of chemically resistant materials. Due to the high reactivity of these resins, radiation curing is preferred.
Undiluted DVK is a solid or waxy substance. Therefore, both reactive and inert diluents are introduced into the composition to provide the viscosity necessary for processing and to increase their reactivity.
The main part of DVA macromolecules consists of epoxy oligomeric blocks of various molecular weights. The higher the molecular weight of such blocks, the higher the strength and elasticity of the resin, but the lower the heat resistance and resistance to solvents.
Compared to polyesters, DVCs are characterized by a lower content of ester groups and vinyl fragments. This leads to an increase in the resistance of these resins to hydrolysis, as well as to a decrease in the temperature of the exotherm peak. Resin shrinkage during curing is reduced. Like polyesters, DVCs have a limited shelf life, which is ensured by the introduction of polymerization inhibitors ("traps" of free radicals) during the resin production process.
Resin production
DVK is obtained by reacting methacrylic or acrylic acids with an oligomeric epoxy resin. The addition reaction of an acid to an epoxide (esterification) is exothermic. As a result of this reaction, free hydroxyl groups are formed on the oligomeric block, but the formation of by-products does not occur (as, for example, during polyesterification, when water is formed). After completion of the reaction or during its course, suitable diluents or polymerization inhibitors are added to the reaction mixture.
Epoxy resins that are used for the production of DVA can be based on bisphenol A (in this case, general-purpose and heat-resistant DVA are obtained), on phenolic-novolac fragments (heat-resistant DVA), and also on a tetrabromo derivative of bisphenol A (fire-resistant DVA). When obtaining DVK with acrylic groups at the ends, oligomeric epoxy blocks based on bisphenol A are usually used as the polymer of the main chain.
Curing
DVC, like unsaturated polyester resins, contains double bonds that react to form crosslinks when cured. This process occurs in the presence of free radicals, which are formed as a result of chemical, thermal or radiation transformations. The curing process proceeding according to the free-radical mechanism includes the stages of initiation (induction period), growth and chain termination. Initiation is a rate-limiting step during which the initiator suppresses the action of polymerization inhibitors. This leads to the reaction proceeding along the double bonds of the vinyl ether, which is part of the macromolecules, and its co-reagent.
Molding Semi-finished products (prepregs) based on DVK for volumetric molding or for sheet plastics are used in direct pressing of fittings for pipes, housings of household appliances, impellers, pumps and car parts. Typically, these prepregs contain approximately equal parts by weight of resin, ground glass fiber, and fillers. They also include: a "hidden" initiator, pigments, anti-adhesion lubricant and thickeners.
TOPIC 4. POLYBUTADIENE RESINS
Polybutadiene resins are high molecular weight, hydrocarbon thermosetting resins. They have excellent electrical properties, significant chemical resistance, sufficiently high thermal stability, low moisture absorption and are easily cured in the presence of peroxide initiators. They can be used for processing by direct compression, injection molding, injection moulding, wet lay-up for laminates and prepregs. Due to the fact that there are many derivatives of polybutadiene, the scope of these polymers is extensive: they are used as modifiers for other resins, for the manufacture of coatings, adhesives and electrical insulating potting compounds.Polybutadiene resins were produced around 1955 and used in Bud-type compounds at Injay Laboratories. The resin that was used in these compounds consisted of a large amount of liquid 1,2-polybutadiene, some styrene-butadiene copolymers, and adducts of the two resins. Since then, similar products have been manufactured by Richardson and Lithium. In 1968, under the brand name "Gistil", they began to produce polybutadiene with a high content of double bonds and a small amount of isocyanate groups at the ends of macromolecules. A certain amount of peroxide initiator was introduced into it.
Now this resin is produced by the firms "Dianachem" and "Nippon Souda" under the trade name "Nisso-RV". This resin is a liquid atactic polybutadiene with a molecular weight of 1000 - 4000, about 90% of the double bonds of which are located in the side chains (vinyl groups).
There are three types of this resin:
type B does not contain terminal functional groups; type G contains hydroxyl groups and type C - carboxyl groups at both ends of the macromolecules. Other polybutadiene resins are now available under the name "Ricone" from Colorado Chemical Specialties. Dienit resins are a mixture of 1,2- and 1,4-polybuta-dnenes (Dienite PD-702, PD-503) or mixtures with monomers-co-reagents, such as vinyltoluene (PM-520, PM-503 ) or styrene-butadiene oligomer (PDPD-753).
Industrial types of polybutadiene resins are usually a mixture of low molecular weight 1,2 - and 1,4-polybutadienes. These isomers differ in the position of the reaction center involved in the polymerization. 1,2-polybutadiene, in which the double bonds are located in the side chains, is more reactive than 1,4-polymers, where the double bonds are in the main chain. Therefore, resins with a high content of 1,2-polybutadiene cure faster and easier, and resins with a significant proportion of 1,4-polymer are usually used to obtain highly elastic materials.
In order to be more convenient to process 1,2-polybutadiene (PBB) resin into composite materials, it should be obtained with a high molecular weight and a narrow molecular weight (MW) distribution. To increase the reactivity of the resin during various chemical transformations, terminal functional groups (for example, hydroxyl, carboxyl, or isocyanate) are introduced into its macromolecules, and mixtures containing polybutadiene and reactive monomers, such as styrene and vinyl toluene, are also prepared. Terminal hydroxyl groups allow reactions with polyurethanes, and carboxyl groups with epoxy groups. Isocyanate-terminated PBBs are mainly used to make electrically insulating potting compounds.
With a high content of vinyl groups (over 85%), polybutadiene resins are easily cured in the presence of peroxide initiators. The reactive terminal functional groups allow the resin to increase in molecular weight even before curing. An increase in MW causes a decrease in the flow of the resin before crosslinking, which causes gelatinization and the appearance of rigid polymer structures.
As a result, a more convenient technological time for processing the resin in the reactor is also achieved. The chain growth step can be controlled (in time) so as to obtain polymers with various properties, from high viscosity liquids to high MW solids. The ability to chain grow is the basis for the widespread use of polybutadiene resins in the production of press compositions, coatings, adhesives, electrically insulating potting compounds and thermoset laminates. The polybutadiene derivatives listed below can be used both as modifiers for other resins and in the production of special laminates
– – –
Resin Curing The similarity of the curing process of polybutadiene resins to the curing of well-known polyester polymers using peroxide initiators makes them extremely useful for composite materials technology.
The curing of the polymer passes through three stages: low temperature gelation, high temperature curing and thermal cyclization. At low temperatures, an increase in the molecular weight and viscosity of the resin occurs.
This can cause gelation and start of curing. High temperature curing starts at 121°C, with dominating reactions at the double bonds of the vinyl groups. During this stage of the process solid products are formed. Thermal cyclization begins at a temperature of ~232°C, and the remaining unsaturated fragments of the polymer substrate react to form a densely crosslinked network.
Below are typical prepreg processing mode data:
Molding temperature, °C
Pressure, MPa
Curing cycle at 77°C for 3.2 mm laminate, min |
Post Curing Period .................. No Chemical Structure and Properties Polybutadiene resins have excellent electrical properties and chemical resistance. The high content of hydrocarbon content and the minimum content of aromatic units are the reason for the low values of dielectric constant and damping factor, as well as excellent chemical resistance. The low content of aromatic fragments explains the high arc resistance, as well as resistance to the formation of conductive traces.
These properties of polybutadiene resins, similar to the behavior of polyethylene, are associated with the resistance of these polymers to the formation of carbon during pyrolysis under high voltage. The absence of ester bonds, which make polyesters vulnerable to acids and bases, explains the hydrophobicity, as well as the resistance of polybutadiene resins to acids and alkalis.
Application of PBB-based CMs Due to the unique combination of excellent electrical properties with chemical resistance, PBB-based CMs have been successfully applied in the design of airborne radar antenna radomes. For operation in the frequency band exceeding the K-band (10.9 - 36.0 GHz), reinforced epoxy glass-reinforced plastics were used, which inadequately meet this purpose due to high dielectric constants (4.5 - 5.0).
This becomes clear if we take into account that the wall thickness of the fairing, as follows from the equation below, is a function of the dielectric constant and the operating wavelength:
n 0 D=, 2(sin 2) 0.5 where d is the wall thickness of the antenna radome; n - integer 0 (n = 0 for a thin wall; n - 1 for a wall with a thickness equal to the half-wave length); 0 - wavelength in free space; - the dielectric constant; - angle of incidence.
Since the wall thickness of the radome must be directly proportional to the effective wavelength but inversely proportional to the dielectric constant, the combination of simultaneously increasing the frequency and using a high dielectric composite creates a wall thickness mismatch problem when longer wavelengths are used.
Obviously, if the wavelength decreases simultaneously and the dielectric constant of the material increases, then it becomes possible to reduce the thickness of the fairing walls. However, the use of thin walls leads to the problem of impact failure, which can be accelerated by severe surface erosion of thin layered structures.
Another problem with higher dielectric materials is the potential for variation in radome wall thickness, resulting in higher production costs or the use of additional materials to ensure accurate "electrical" thickness. When using antennas on aircraft and ships, additional requirements are imposed on the CM, from which the radomes are made: they must have stable properties in a wide range of temperatures and in conditions of high humidity. Strict material requirements associated with high operating frequencies and difficult environmental conditions are not easily met using conventional composite materials. However, these requirements can be met more fully when using materials based on polybutadienes.
When preparing prepregs, resins are cured in the presence of a peroxide initiator. Despite the excellent processability of this CM and the ease of curing, which is completed in one stage in 2 hours at a temperature of 177 °C, its low mechanical properties in the transverse direction limit its use as a structural material. This disadvantage is possibly associated with a high density of intermolecular crosslinks, which leads not only to brittleness, but also to low adhesion of the binder to carbon fibers.
When obtaining polybutadiene layered plastics for structural purposes, various reinforcing fibers are used: glass, quartz and aramid ("Kevlar-49"). Composites reinforced with Kevlar-49 fiber with a volume fraction of 60% are suitable for the manufacture of radar antenna radomes. To improve some mechanical properties of the material, especially the tensile strength in the transverse direction and interlaminar shear, the adhesive properties and wettability of the Kevlar-49 fiber need to be improved.
An additional requirement when using these materials for the manufacture of radar antenna radomes is low moisture absorption.
Storage Polybutadiene resins do not require any special storage conditions compared to the usual ones associated with the use of volatile, flammable organic solvents such as heptane or toluene. When stored at temperatures of 0, 20 or 35 °C for 10 weeks, there is no noticeable change in viscosity or separation of the solution. However, longer storage at temperatures above 35°C should be avoided due to the tendency of the solution to gel.
EPOXIES Epoxy resins are one of the best binders for a wide variety of fiber composites for the following reasons:
Good adhesion to a large number of fillers, reinforcing components and substrates;
A variety of available epoxy resins and curing agents, allowing to obtain after curing materials with a wide combination of properties, meeting various requirements of the technology;
No release of water or any volatile substances during the chemical process and slight shrinkage during curing;
Chemical resistance and good electrical insulating properties.
The main component of epoxy binders is a mixture of oligomeric products with epoxy groups in the end units (epoxy resins).
They are received:
the interaction of epichlorohydrin with dihydric (less often, polyhydric) alcohols or phenols to form diglycide oxyethers CH2-CH-CH2Cl + HO-R-OH CH2-CH-CH2-O-R-(-O-CH2-CH(OH)-CH2-O- RO O)-O-CH2-CH-CH2 \ / O or CH2-CH-CH2Cl + H2N-C6H4-NH2 \/ O or CH2-CH-CH2Cl + HO-C6H4-C (CH3) 2-C6H4-OH bisphenol A \/ O The most common resins are those obtained from epichlorohydrin and diphenylolpropane (bisphenol A) (ED type resins) or from epichlorohydrin and methylolphenol polycondensation products (epoxyphenolic resins EF, EN). Recently, resins from epichlorohydrin and aniline (EA resin), diaminodiphenylmethane (EMDA) have been used.
Application Epoxy resins are used in the production of various composite materials and structural parts. They are also used as encapsulating and sealing compounds, press powders and for the manufacture of adhesives.
Epoxy resins are very resistant to acids, alkalis and moisture, do not deform when heated to high temperatures, have low shrinkage and high volume resistivity. Epoxy resins can be used not only to protect materials from the effects of the environment, but also to glue parts together. In the electronics industry, for example, epoxy resins are used for encapsulating welded modules, casting transformer and motor windings, and also for sealing electrical cable joints.
Since the Second World War, epoxy resins have been used to make tooling (for example, molds used in sheet stamping or models in the manufacture of parts). Reinforcing fillers in the form of particles or fibers are easily introduced into the resin, reducing its cost and increasing dimensional stability. The possibility of replacing metals with epoxy resins is due to two factors: cost-effectiveness in production and speed (without large material costs) of modification. In addition, these resins retain their shape and dimensions well, have high mechanical properties and low shrinkage, which makes it possible to manufacture parts with small tolerances from them.
Molding Epoxy molding compounds (powdery, partially cured mixtures of resin and hardener that flow when heated) are used to produce all kinds of structural parts. Fillers and reinforcing agents are easily introduced into epoxy resins, forming a molding mass. Epoxy resins provide low shrinkage, good adhesion to fillers and reinforcing agents, chemical stability, good rheological properties.
Bonding Of all known polymeric materials, epoxy resins have the highest adhesive strength. They are used to impregnate a variety of substrates with minimal shrinkage. Therefore, these resins can be used to bond many dissimilar materials. In addition, they can be cured at different temperatures and at different speeds, which is very important in the industrial production of adhesives.
Fabrication of fiber wound CMs and as laminates One of the most important applications of epoxy resin or binder is in the production of laminates and fiber wound composites for the manufacture of structural parts. Such parts are used in various industries, including aircraft construction, space and military equipment. Laminates are also used in the electronics industry for the manufacture of printed circuit boards. Tanks and pipes made of epoxy composites are widely used in the chemical and petrochemical industries.
Epoxy resins can be used in a variety of processes: wet winding fiber or "wet" forming laminates, dry winding or laying layers with pre-impregnation of fiber strands, fabrics or tape (in the form of prepregs). In general, epoxies are more expensive than most other resins, but their excellent performance properties often make them more profitable in the long run.
Amine Curing of Resins The vast majority of epoxy oligomers are either viscous liquids or low melting solids readily soluble in ketones, ethers, and toluene.
Epoxy oligomer hardeners are divided into two large groups according to the mechanism of action:
Cross-linking hardeners contain functional groups that chemically interact with the functional groups of the epoxy oligomer;
Catalytic hardeners cause the formation of a spatial network structure by polymerization of epoxy groups.
Cross-linking hardeners contain amino, carboxyl, anhydride, isocyanate, hydroxyl and other groups in their molecules.
Amine type hardeners are used for curing in the operating temperature range of 0-150 °C. As aliphatic amines, 1,6-hexamethylenediamine and polyethylenepolyamines of the general formula H2N(CH2CH2NH),CH2CH2NH2, where n = 1-4, are widely used, having high activity even at a temperature of 20 °C.
As aromatic amines, m-phenylenediamine, 4,4"-diaminodiphenylmethane, 4,4"-diaminodiphenylsulfone are used. Aromatic amines are less active than aliphatic ones, and they cure at temperatures of 150 °C and above.
Dicyandiamine is widely used as an amine-type hardener.
Dicyandiamine practically does not react with epoxy oligomers at room temperature, but quickly cures them at elevated temperatures (150 °C and above).
For complete crosslinking of the epoxy resin, the ratio between the number of hydrogen atoms in the amino groups of the hardener and the number of epoxy groups in the resin must be 1:1. The reaction between aliphatic amines and epoxy groups proceeds at room temperature. In the case of using harsh aromatic amines, heating is necessary. The chemical bond between carbon and nitrogen atoms that occurs when the resin is “crosslinked” with amines is resistant to most inorganic acids and alkalis. However, this bond is less stable to organic acids than the intermolecular bonds formed by hardeners of other classes. In addition, the electrical insulating properties of "amino-cured" epoxies are not as good as with other curing agents. Perhaps this is due to the polarity of the hydroxyl groups formed during curing.
Isocyanate hardeners easily react with hydroxyl groups of epoxy oligomers even in the cold (=20 °C). At high curing temperatures (180-200 °C), the reaction of the isocyanate group with the epoxy group is possible with the formation of an oxazolidone cycle. As isocyanates, 2,4- and 2,6-toluene diisocyanates, hexamethylene diisocyanate and prepolymers based on them with terminal isocyanate groups are used.
For curing epoxy oligomers, phenol-formaldehyde oligomers of both novolac and resole types are widely used. Novolacs cure epoxy oligomers by reacting phenolic hydroxyls with epoxy groups at 150-180°C, and in the presence of catalysts (tertiary amines) at 80°C. In the case of resols, the hydroxymethyl groups of resols react with the secondary OH groups of the epoxy oligomers and, in addition, can alkylate the aromatic rings of the epoxy oligomers.
Catalytic hardeners catalyze the polymerization of epoxy groups by cationic and anionic mechanisms.
Cationic polymerization is initiated by Lewis acids - BF3, BF30(C2H5)2, SnCl4, etc.
Anionic polymerization is initiated by alkali metal hydroxides and alcoholates, as well as tertiary amines such as triethanolamine and 2,4,6-tris(dimethylaminomethyl)phenol.
In anionic polymerization in the presence of tertiary amines, the active site is formed by the co-reaction of the amine, epoxy site and alcohol according to the O OH scheme. Aliphatic tertiary amines are usually cold curing hardeners. Recently, imidazoles (in particular, 2-ethyl-4-methylimidazole) have been successfully used as Lewis base-type hardeners, which impart increased heat resistance to polymers. Storage of amine hardeners usually does not cause any special problems. However, they can cause skin irritation in some people and should be handled with care.
Curing resins with acid anhydrides As acid hardeners, cyclic aldehydes of carboxylic acids, such as phthalic, maleic, as well as trimellitic (TMA), pyromellitic (PMA), benzophenonetetracarboxylic acid anhydride (ABTC) have found the greatest use. Curing with carboxylic acid anhydrides is carried out at 120- 180 °C.
The storage of these hardeners requires special care to prevent their decomposition by atmospheric moisture. To ensure complete curing, the reaction is carried out under heating. Often a small amount of accelerator is added to speed up the curing process, which is extremely slow. There are also anhydrous hardeners that react with the resin when heated above 200°C. Acid anhydrides react with epoxy resins to form esters. For this reaction to occur, anhydride ring opening is required. A small amount of proton-containing substances (for example, acids, alcohols, phenols and water) or Lewis bases promotes ring opening.
The ester group formed as a result of curing is resistant to the action of organic and some inorganic acids, but is destroyed by alkalis. The resulting materials have greater thermal stability and better electrical insulating properties than when using amine hardeners.
Lewis acid catalytic curing Only one of the Lewis acids, boron trifluoride, is widely used as a curing agent for epoxy resins. When added in small amounts to pure epoxy, this hardener acts as a catalyst for the cationic homopolymerization of the resin to polyether. Boron trifluoride causes a very rapid exothermic polymerization occurring in a few minutes. Therefore, when curing a large amount of resin, to maintain room temperature in the mass, it is required to block it using a special technology. When combined with monoethylamine (MEA) to form the BF3-MEA complex, boron trifluoride converts at room temperature into a latent curing agent. At temperatures above 90°C, it becomes active and causes rapid curing of the epoxy resin, accompanied by a controlled release of heat. When receiving prepregs, which are often stored for weeks before processing, the use of a latent hardener is absolutely essential.
Epoxy resins containing the BF3-MEA complex are widely used for sealing, in the manufacture of tooling, laminates and winding products.
Some limitation here is the found instability of prepregs and curing compositions containing VG3MEA to the action of moisture.
Accelerators Accelerators are added to resin and hardener mixtures to speed up the reaction between them. They are introduced in small non-stoichiometric amounts, which are selected empirically, guided by the properties of the resulting material. Some of the tertiary amines - curing catalysts - can also be accelerators for a number of systems. Most often they are used to increase the curing rate of epoxy resins with acid anhydrides. For this purpose, tin octanate, which is a Lewis acid, is used. In some cases, it allows curing at room temperature.
Cured Epoxy Resins Some generalizations can be made regarding the relationship between the chemical structure and properties of cured epoxy resins:
The more aromatic rings are included in the composition of the epoxy resin, the higher its thermal stability and chemical resistance;
When using aromatic hardeners, more rigid and durable materials are formed than in the case of aliphatic agents, however, the increased rigidity of such systems reduces molecular mobility and thereby complicates the interaction between the reactive groups, and curing in this case is carried out at elevated temperatures;
Reducing the density of intermolecular "crosslinks" can lead to an increase in the strength of the material, due to an increase in elongation at break;
Reducing the density of "crosslinks" can also lead to a decrease in resin shrinkage during curing;
Increasing the density of "crosslinks" leads to an increase in the chemical resistance of the cured material;
An increase in the density of "crosslinks" leads to an increase in the thermal degradation temperature (and glass transition temperature Tc), however, too high a density of "crosslinks"
reduces the fracture deformation (increased brittleness);
When replacing aromatic fragments of molecules with aliphatic or cycloaliphatic ones, which is not accompanied by a change in the number of "crosslinks" in the system, the elasticity and elongation of the cured resin increase;
Acid anhydride cured epoxies perform better in acid service than in alkaline service.
Due to the fact that epoxy resins are viscoelastic materials, their properties depend on both temperature and test duration (speed, frequency).
Properties of epoxy resins cured by special methods.
When using specifically cured epoxy systems, some limitations must be considered. For example, in the case of manufacturing large parts that are inconvenient for heating, and thick-walled parts, where thermal stresses should be minimal, it is inappropriate to use systems that require high-temperature curing. In these cases, systems with low temperature hardeners are used. These compositions include epoxy resins cured by the action of aliphatic amines. Curing such compositions at room temperature results in materials with excellent properties, further improved with low heat. Of course, these resins cannot be used at high temperatures.
Epoxy oligomers and polymers are used in various fields of technology due to the successful combination of a simple processing technology with high physical and mechanical properties, heat resistance, adhesion to various materials, resistance to various media, and the ability to cure at atmospheric pressure with low shrinkage. So, they are widely used in the production of high-strength structural materials, in rocket and space technology, aviation, shipbuilding, mechanical engineering, electrical engineering, radio electronics, and instrument making.
Epoxy oligomers and polymers are widely used as matrices for the production of carbon plastics, which are characterized by a combination of high strength and rigidity with low density, low temperature coefficient of friction, high thermal and electrical conductivity, wear resistance, resistance to thermal and radiation effects. Coking and pyrocarbon epoxy carbon plastics are resistant to thermal and thermal oxidative degradation, have high strength characteristics, and have good heat-shielding properties.
Epoxy resins are good matrices for making fiberglass. In addition to glass fibers and glass fabrics, quartz fibers and fabrics, boron carbon fibers, silicon carbide and other inorganic fibers are used.
In addition to inorganic fibers, to obtain reinforced epoxy plastics, fibers from organic polymers are used, in particular, high-strength synthetic fibers from poly-p-phenylene terephthalamide and other aramids.
Due to good adhesion to glass, ceramics, wood, plastics, metals, epoxy oligomers and polymers are widely used in the production of adhesives, hot and cold curing compounds.
Epoxy oligomers are used to seal and encapsulate various parts in order to protect them from the environment.
In electrical engineering, epoxy oligomers are used to fill the windings of transformers and motors, to seal the joints of electrical cables, etc.
TOPIC 6. HEAT-RESISTANT RESINS
Heat-resistant resins are linear or cross-linked heteroaromatic polymers that have a high glass transition temperature and are able to withstand prolonged heating in air above 300 ° C without noticeable changes in the structure.Despite the process of thermal-oxidative degradation, which inevitably proceeds under these conditions, the decomposition of such polymers proceeds relatively slowly. In addition, it is assumed that the fragments into which these polymers break down are relatively stable, which increases the "life" of the material at elevated temperatures.
The key point in obtaining heat-resistant resins is the synthesis of polymers containing a large number of heteroaromatic fragments. These fragments, containing the minimum number of hydrogen atoms capable of being oxidized, can absorb thermal energy. Unfortunately, the same elements of the chemical structure that determine the thermal and oxidative stability of such resins lead to serious difficulties, and often even to the impossibility of their processing into the desired products.
In the 1960s, a number of heteroaromatic polymers were synthesized, which, according to thermogravimetric analysis (TGA), had good thermal and oxidative stability at elevated temperatures. However, attempts to use these polymers as binders for improved composite materials have been either unsuccessful or not economically viable.
Therefore, in the early 1970s, the future of heat-resistant polymer binders looked very vague and uncertain. It seemed that this useful class of materials would remain a "laboratory curiosity." However, the development of the chemistry of polyimide polymers in 1972-74. not only revived interest in them and caused new developments in the field of heat-resistant binders, but also made it possible to practically realize many of the potential possibilities of these binders. Currently, polyimide fibrous composite materials are used as structural materials operating at a temperature of about 300 °C or three-dimensional (spatial mesh).
The main disadvantage of composite materials based on high-molecular-weight polyimides is their high porosity, which sharply limits the possibilities of effective practical application of these materials under conditions of simultaneous exposure to high mechanical loads, high temperatures, and an oxidizing atmosphere.
Therefore, it seems more appropriate to use the initial fusible oligomeric imides capable of curing by the polymerization reaction, since polymerization is not accompanied by the release of volatile by-products, leading to high porosity of the resulting materials. Of greatest importance are polymerizable oligomeric imides containing maleicimide and engroups at the ends of the chains.
These requirements are largely satisfied by bismaleimyls obtained by the interaction of diamines of various structures and maleic anhydride. The double bond in bis-maleimides is electron-deficient due to the proximity to the carbonyl groups of the imide cycle; therefore, bis-maleimides easily polymerize when heated above the melting point, forming three-dimensional polymers.
RETURNABLE WASTE OF AN INDUSTRIAL ENTERPRISE Waste is generated in the course of economic activity of almost all enterprises. Due to the fact that the amount of waste directly affects ... " FEDERAL AGENCY FOR EDUCATION MOSCOW STATE CONSTRUCTION UNIVERSITY PROGRAM OF discipline _Economic evaluation of investments_ "ECONOMY OF THE PUBLIC SECTOR" It is shown that the problem of interaction between market mechanisms and state regulation should discuss ... "candidate of philosophical sciences, junior researcher of the department of sociology and psychology N ... "contributors, Dean Witter Department of Finance, who receive a short-term Stanford degree ... "N. V. Mikhailova Minsk State Higher ... "
2017 www.site - "Free electronic library - various materials"
The materials of this site are posted for review, all rights belong to their authors.
If you do not agree that your material is posted on this site, please write to us We will remove it within 1-2 business days.